by Piter Kehoma Boll
Here is a list of species described this month. It certainly does not include all described species. You can see the list of Journals used in the survey of new species here.
Bacteria
- 6 new actinobacteria: Arthrobacter bussei; Micromonospora pelagivivens; Georgenia wutianyii, Georgenia yuyongxinii; Streptomyces tailanensis; Streptomyces cahuitamycinicus;
- 11 new bacteroids: Antarcticibacterium arcticum; Pedobacter cryotolerans, Pedobacter cryophilus, Pedobacter frigiditerrae, Pedobacter psychroterrae, Pedobacter hiemivivus, Pedobacter frigidisoli, Pedobacter frigoris, Pedobacter psychrodurus, Pedobacter polaris; Hymenobacter lutimineralis;
- 1 new cyanobacterium: Nostoc neudorfense;
- 5 new firmicutes: Bacillus salinus; Bacillus fungorum; Vagococcus xieshaowenii; Cohnella fermenti; Streptococcus caledonicus; Lactobacillus enshiensis;
- 2 new fusobacteria: Oceanivirga miroungae; Streptobacillus canis;
- 2 new planctomycetes: Roseimaritima sediminicola; Aureliella helgolandensis;
- 31 new proteobacteria: Novosphingobium silvae; Pseudomonas piscis; Aquabacterium lacunae; Marinobacter denitrificans; Roseibium aestuarii; Methylobacterium nonmethylotrophicum; Chitinasiproducens palmae; Halomonas montanilacus; Halomonas piezotolerans; Ruegeria sediminis; Janthinobacterium violaceinigrum, Janthinobacterium aquaticum, Janthinobacterium rivuli; Methylotenera oryzisoli; Arenimonas fontis; Haemophilus seminalis; Yersinia canariae; Chimaeribacter arupi, Chimaeribacter coloradensis, Chimaeribacter californicus; Nocardioides euryhalodurans, Nocardioides seonyuensis; Vitreimonas flagellata; Antarcticimicrobium sediminis; Sulfurimonas xiamenensis, Sulfurimonas lithotrophica; Microbulbifer harenosus; Lysobacter lacus; Roseovarius arcticus; Azoarcus nasutitermitis, Azoarcus rhizosphaerae; Mesorhizobium alexandrii;
Archaeans
- 1 new euryarchaeote: Salinibaculum litoreum;
SARs
- 6 new ciliates: Clevelandella lynni, Nyctotherus galerus; Cyrtohymena seorakensis; Tetrahymena acanthophora, T. dugesiae, and T. nigricans;
- 3 new dinoflagellates: Pachena leibnizii, P. abriliae, P. meriddae;
- 1 new apicomplexan: Eimeria riparii;
- 6 new ochrophytes: Pinnularia baetica; Gomphonema chemeron; Sellaphora mayrii; Sellaphora wangii; Cymbella stigmacentralis, C. compactiformis;

Plants
- 2 new rhodophytes: Sirodotia assamica; Calidia pseudolobata;
- 4 new chlorophytes: Chlorocladiella cochlea, C. erecta, C. medogensis, C. pisformis;
- 1 new pteridophyte: Lepisorus youxingii;
- 3 new magnoliids: Myristica trobogarii; Phoebe hekouensis; Cryptocarya kaengkrachanensis;
- 13 new monocots: Allium nerimaniae; Epidendrum machinense; Chascolytrum serranum; Liparis mai; Pseudoliparis sp.; Gastrodia gunatillekeorum; Platanthera jiuwanshanensis; Chamaedorea vanninii; Bulbophyllum trongsaense; Alocasia lihengiae; Sciaphila kozushimensis; Cheirostylis barbata; Monstera guzmanjacobiae; Aspidistra jiangjinensis;
- 35 new eudicots: Salvia rhizomatosa; Melastoma malabituin; Rinorea gemmulata; Lampranthus alboroseus; Stellaria pentastyla; Sonerila sulpheyi; Acridocarpus taitensis; Stellaria procumbens; Myrceugenia joinvillensis; Myrcia lucasae; Bauhinia corifolia; Hieracium richianum; Wahlenbergia itatiaiensis; Euphorbia pisima, E. steelpoortensis; Centaurea akroteriensis; Lythrum netofa; Hydrocotyle simulans; Begonia enoplocampa; Argostemma separatum; Begonia vasconselosiana; Eranthis tanhoensis; Pinguicula rosmarieae; Capsicum carassense; Ipomoea nivea, I. apodiensis, I. calcicola, I. pochutlensis, I. zacatecana, I. ramulosa; Sorbus gongshanensis; Onosma fuyunensis; Dischidia phuphanensis; Fothergilla milleri; Miconia lucenae;

Fungi
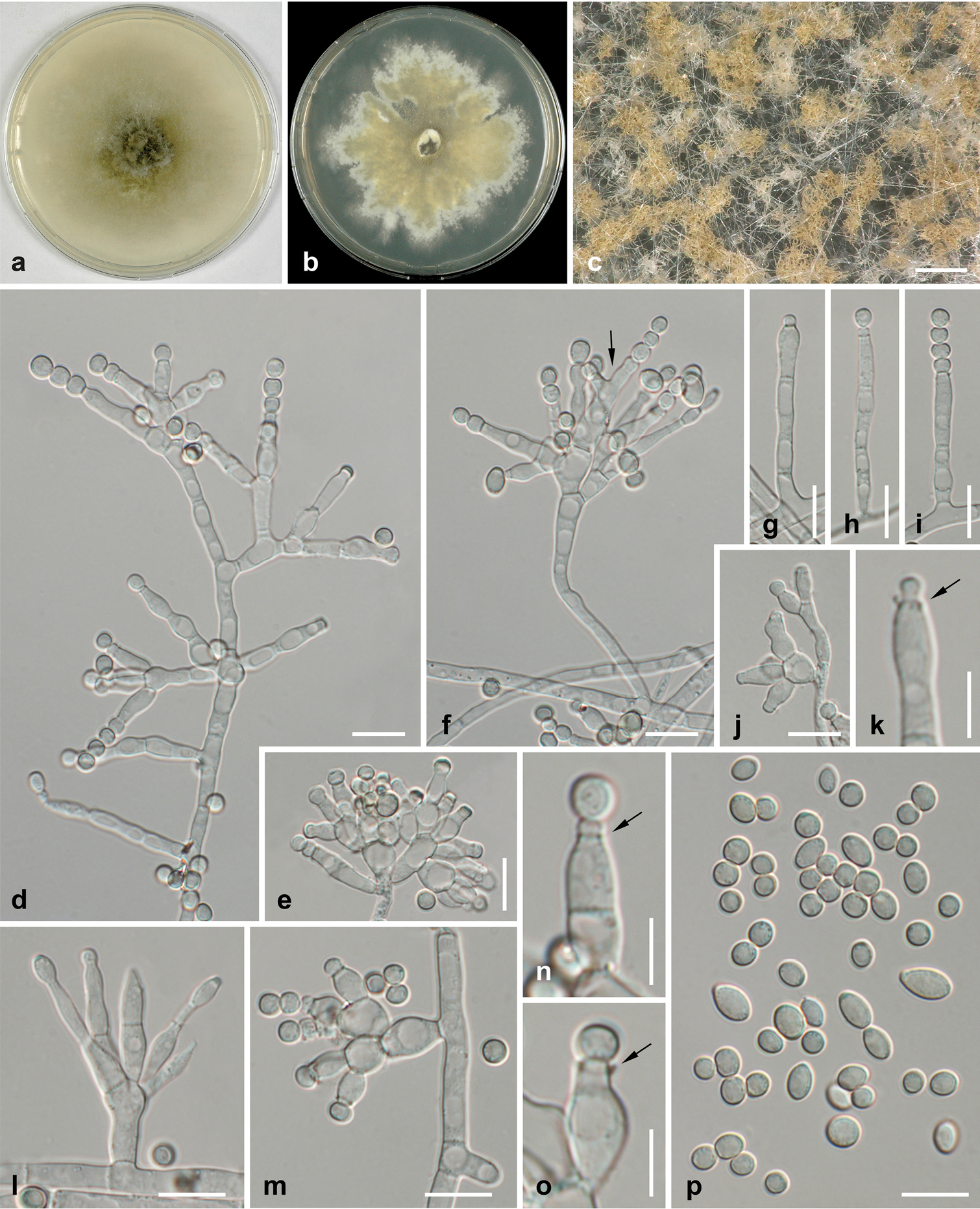
- 37 new ascomycetes: Leptosillia cordylinea; Xylaria acericola; Phaeosphaeriopsis omaniana; Leucaenicola osmanthi; Melanconis larissae, M. pacifica; Arboricolonus simplex, Cadophora africana, C. prunicola, C. ramosa, Proliferodiscus ingens, Minutiella pruni-avium; Ascochyta benningiorum, Didymella degraaffiae, D. kooimaniorum, Juxtiphoma kolkmaniorum, Nothophoma brennandiae, Paraboeremia rekkeri, P. truiniorum, Stagonosporopsis stuijvenbergii, S. weymaniae, Vandijckomycella joseae, V. snoekiae, Xenodidymella weymaniae; Tingoldiago hydei, T. clavata; Ochraceocephala foeniculi; Zygotorulaspora cariocana; Wickerhamiella osmotolerans, Wickerhamiella tropicalis; Microascus aculeatus, M. spinosporus; Beauveria mimosiformis; Didymella corylicola; Colletotrichum eriobotryae; Trichoderma azevedoi, Trichoderma peberdyi;
- 28 new basidiomycetes: Gyroporus madagascariensis, Gyroporus borealis, Gyroporus smithii; Tylopilus jiangxiensis; Melampsora salicis-michelsonii; Stephanospora xibalba; Lactifluus umbilicatus, Lf. venosellus; Xanthagaricus siamensis; Heteroradulum yunnanensis; Wrightoporia srilankensis; Trametes parvispora; Lyomyces bambusinus, L. cremeus, L. macrosporus and L. wuliangshanensis; Chroogomphus pakistanicus, C. pruinosus; Moniliophthora mayarum; Multiclavula petricola; Puccinia dimorphothecae-cuneatae, P. feliciicola, Uredo myricae, Uromyces hessii; Marantokordyana oberwinkleriana, M. boliviana; Peniophorella fissurata, P. yunnanensis;

Sponges
- 5 new calcareans: Clathrina fakaravae, Clathrina huahineae, Ernstia variabilis, Leucascus digitiformis, Leucandra tahuatae;
- 3 new demosponges: Acantorna tahoma; Stellettinopsis capixaba, Stellettinopsis baiana;
- 1 new hexactinellid: Farrea cordelli;

Cnidarians
- 8 new hydrozoans: Cladocarpus asymmetricus, C. partitus, C. pennatus, Lytocarpia fragilis, L. pilosa, L. pseudoctenata, L. subtilis, Macrorhynchia spiralis;
- 3 new anthozoans: Chrysogorgia dendritica, Chrysogorgia fragilis, Chrysogorgia gracilis;
- 1 new myxozoan: Myxobolus adrianoi;
Flatworms
- 1 new rhabdocoel: Temnocephala ivandarioi;
- 5 new trematodes: Cryptocotyle dominicana; Gaharitrema droneni; Brachylaima succini; Allocreadium khankaiensis; Skrjabinopsolus nudidorsalis;
- 9 new cestodes: Emberizotaenia aeschlii, Anonchotaenia kornyushini, Biuterina jensenae, Raillietina hymenolepidoides, R. mahnerti; Phoreiobothrium sorrahcola; Scyphophyllidium timvickiorum, Clistobothrium amyae, Clistobothrium gabywalterorum;
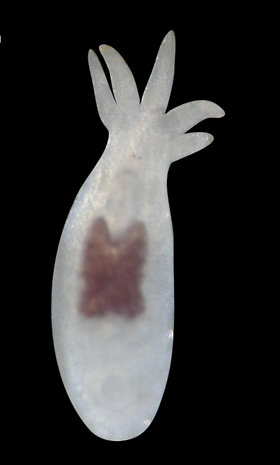
Gastrotrichs
- 1 new species: Acanthodasys australis;
Rotiferans
- 1 new acanthocephalan: Moniliformis necromys;
Mollusks
- 29 new gastropods: Dendronotus jamsteci, D. zakuro D. bathyvela; Bythinella kyriaki, B. gregoi, B. pesici, B. klimaensis, B. righaensis, B. radomani, B. kwanti, B. petrosensis, B. liandinaensis, B. konstadinensis, B. olymbosensis, B. dimitrosensis; Amphorina viriola, A. andra; Parmacochlea balios, P. furca, Thularion stanisici, Antiquarion boondah, A. chooreechillum, A. evelynensis, A. limbus, A. occultus, A. ravenshoe, A. swausi, Limpidarion kukuyulangi; Peronia persiae;
- 1 new bivalvian: Cardiomya minerva;
Annelids
- 18 new polychaetes: Eunice conchinna, E. confusus, E. cultrifera, E. upoloae, Leodice diversidentata, L. jimedwardsi, L. tasmaniae; Spirobranchus sinuspersicus; Manayunkia occidentalis; Enchiridium daidai; Parougia spp.;
- 2 new clitellates: Aberrantidrius mihaljevici, Stylodrilus tofaceus;
Bryozoans
- 13 new species: Parasmittina acondylata, Metroperiella cotoensis, Microporella tonkinensis, Rhynchozoon setiavicularium, R. latiavicularium, Disporella phaohoa; Hippothoa catophilia, Plesiothoa densa, P. gerroa, Neothoa bilderbacki, Jessethoa ausubeli, Antarctothoa pansa, A. ballia;
Nematodes
- 12 new chromadoreans: Cloacina woworae, C. beveridgei, Cervonemella kaimanaensis; Sphaerolaimus callisto, Sphaerolaimus ganymede, Sphaerolaimus io; Raphidascaris (Ichthyascaris) spinicauda, Raphidascaris (Ichthyascaris) fasciati, Raphidascaris (Ichthyascaris) nudicauda, Raphidascaris (Ichthyascaris) euani, Raphidascaris (Ichthyascaris) elopsis; Acrostichus ziaelasi;
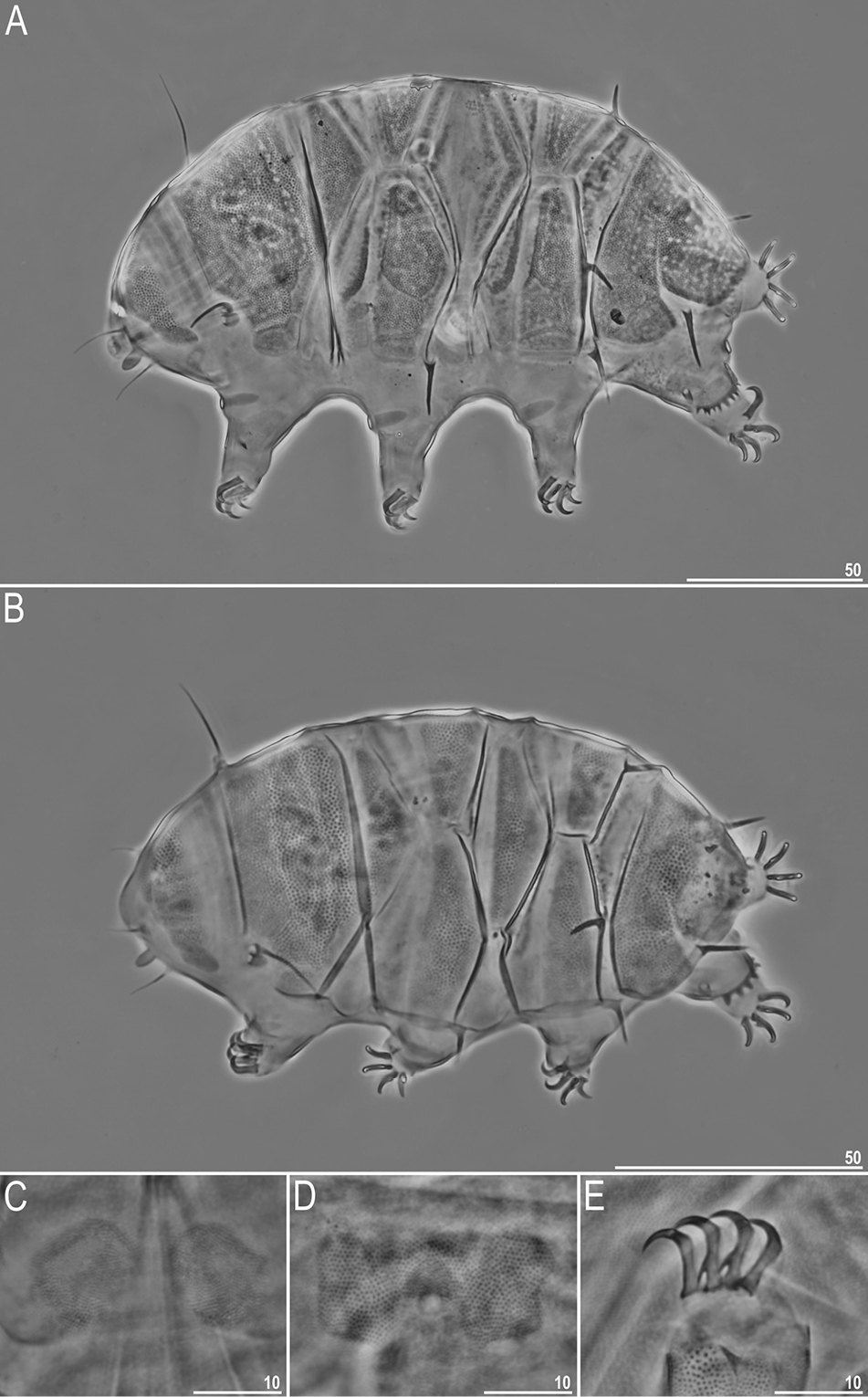
Tardigrades
- 6 new species: Moebjergarctus clarionclippertonensis; Macrobiotus wandae; Macrobiotus engbergi, Tenuibiotus zandrae; Cyaegharctus kitamurai; Echiniscus masculinus;
Chelicerates
- 3 new pycnogonids: Pycnogonum (Nulloviger) granulatum; Labrunoides vibrissa; Cilunculus tricuspis;
- 19 new mites: Aetacarus accipiter, Capitolichus campoflicker, Coraciacarus cabure, Coraciacarus peixefrito, Gabucinia neotropica, Hieracolichus caboclo, Hieracolichus falcon, Piciformobia adjuncta, Proaposolenidia bicolor Proaposolenidia plumbea, Tocolichus toco; Phyllodispus tenuisetus, Promicrodispus bisetus, Premicrodispus (Premicrodispus) novaezealandicus, Premicrodispus (Premicrodispulus) secundus; Perscheloribates paracuriosus, Perscheloribates parakontumensis; Bryobia (Allobia) syriensis; Aceria ajabshiriensis;
- 5 new harvestmen: Ampliphallus chimalapaensis, Guelaguetzia cuicateca, G. serrana; Quindina hermesi, Q. discolor;
- 4 new pseudoscorpions: Parobisium motianense, P. qiangzhuang, P. sanlouense, P. tiani;
- 27 new spiders: Ixchela panchovillai, Ixchela zapatai; Khorata nani, Khorata yuhaoi; Nyetnops naylienae, Nyetnops lachonta, Nyetnops buruti; Desis sp.; Maratus azureus, Maratus constellatus, Maratus inaquosus, Maratus laurenae, Maratus noggerup, Maratus suae, Maratus volpei; Bosselaerius hyrcanicus, B. tajikistanicus, Phrurolithus azarkinae; Segestria nekhaevae, S. shtoppelae, S. fengi; Pholcus jingnan, Pholcus longlin; Manzuma botswana, M. petroae, M. tanzanica; Opopaea kanpetlet, O. zhigangi;

Myriapods
- 38 new diplopodans: Catharosoma pedritense, Catharosoma promatense, Catharosoma ibirapuitense; Taiyutyla tillamook, Taiyutyla acuphora, Taiyutyla amicitia, Bollmanella bombus, Bollmanella washingtonensis, Bollmanella leonardi, Brunsonia pulchra, Brunsonia digitata, Brunsonia wenatchee, Brunsonia chelanoparva, Brunsonia chelanomagna, Brunsonia selwayana, Brunsonia benewah, Calityla siskiyou, Calityla ubicki, Calityla trinitaria, Calityla essigi, Calityla humboldtensis, Ovaskella ovaskae, Ovaskella sinuosa, Karagama ladybird, Complicatella pectenifera, Complicatella neili, Bifurcatella olympiana, Bifurcatella hoh, Bifurcatella angulata, Bifurcatella pacifica, Bifurcatella germania, Bifurcatella uniclada, Bifurcatella inflata, Bifurcatella hobo, Loomisiella evergreen, Loomisiella pylei; Trisaria rex, T. olympia, T. washingtonensis;
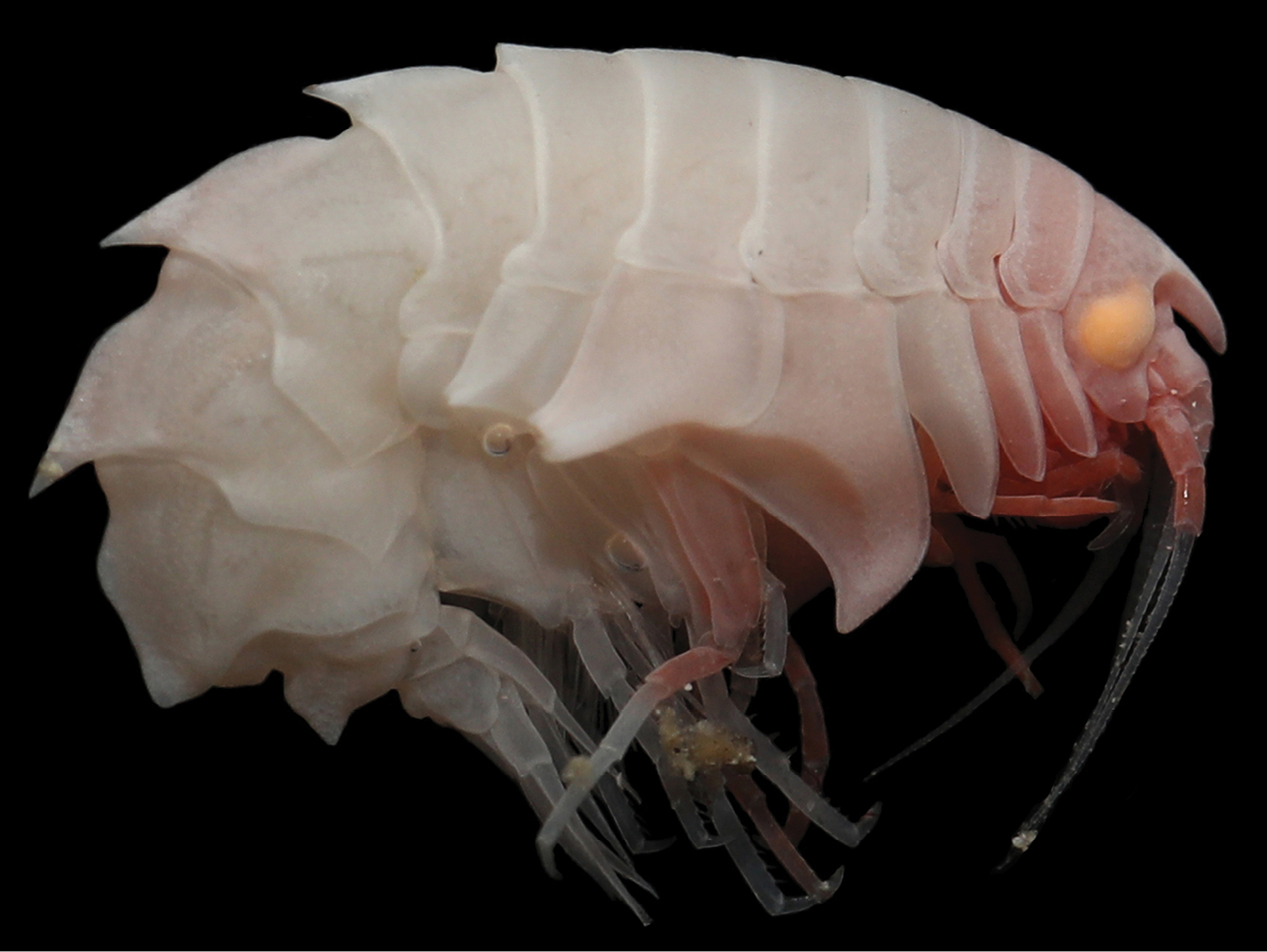
Crustaceans
- 1 new cladoceran: Alpinalona fornshelli;
- 2 new tanaids: Unispinosus sp., Portaratrum sp.;
- 11 new amphipods: Eurythenes plasticus; Gondwanorchestia tristanensis; Rhachotropis reiwa; Eusirus bonnieri; Sigalopella ammophila, S. nigrofemoralis, S. armeniaca, S. rufifemoralis; Paraproto murrayae; Liljeborgia associata; Epimeria liui;
- 2 new decapods: Alpheus samudra; Fragillianassa joeli;


Hexapods
- 2 new diplurans: Remycampa herbanica, Spaniocampa relicta;
- 3 new collembolans: Semicerura bryophila, S. draconis; Furculanurida bistribus;
- 1 new odonate: Telebasis rojinegra;
- 13 new ephemeropterans: Thraulodes ludmilae, Th. panamensis, Th. viviparus, Th. fascipennis, Th. flavus, Th. niger, Th. nigrabdominalis, Th. nigripes, Th. nigrotibialis, Th. alboniger; Procerobaetis leptobranchius, P. petersorum, P. freitagi;
- 5 new dictyopterans: Amitermes bandeirai, Amitermes lilloi; Chlidonoptera roxanae; Rustitermes boteroi; Didymocorypha libaii;
- 13 new orthopterans: Prionotropis xausi; Xizicus (Axizicus) furcus, Xizicus (Eoxizicus) gaoligongshanensis; Nahlaksia hainanensis; Paradoxitettigia longicaudica; Caloxiphus chapulhuacan, C. cuicani; Mecopoda crescendo, M. himalaya, M. tibetensis, M. confracta, M. synconfracta, M. minor;
- 4 new thysanopterans: Ctenothrips parisae; Thrips reunionensis; Apelaunothrips moundi; Hoplothrips sp.;
- 4 new plecopterans: Amphinemura bifascia, A. bicornata; Hemacroneuria baotianmana; Sinonemura balangshana;
- 2 new psocopterans: Liposcelis aleksandrowiczi; Pedicinus gabonensis;
- 22 new hemipterans: Scaphomonus naejangsanus; Alnetoidia (Alnetoidia) jejudoensis; Elanela colombiana, Elanela ecuatoriana; Metopolophium arcticum, Metopolophium taimyricum; Minutaleyrodes andamanensis,d Minutaleyrodes whisper; Aphelonotus schuhi; Uroleucon australe; Hishimonus adi; Pulvinaria pistaciae; Cladonota (Falculifera) rex; Heissiella donguri; Flatfronta dibangi, F. uttara; Daochia fenestrata; Sivaloka arcuata, Sivaloka trigona, Kodaianella furcata; Hornylia obtusipetala; Anomoneura taiwanica;
- 90 new coleopterans: Ochraethes nevadensis, Psyrassa audureaui, Oxylymma rileyi; Phytoecia (Neomusaria) kazaryani, Phytoecia (Parobereina) kashanica; Ceratophyus amdoensis; Pseudoechthistatus rugosus; Acoma chihuahuaensis, A. eusexfoliata, A. nonglabrata, A. pararobusta; Neocompsa bravo; Exomis deqinensis, E. huangi, E. pubescens, E. pubipennis, E. tanae, E. viridis; Thopeutica wiesneri; Scythropopsis granitica, Apeba danielvlasaki, Giesberteclipta unicolor, Anelaphus rotundus, Euderces hefferni; Indenicmosoma paicum; Bothynus araya, Bothynus condacki; Thermistis annamensis; Trichocircus mexicanus, Protandroconnus akeratophorus; Nazeris lushanensis, N. cuscutus, N. baoxingensis, N. megalocephalus, N. fulongensis, N. tarandoides; Megarthrus chujiao; Hydraena (Hydraenopsis) josefinae, H. (H.) pernambucana; Orphnus brevialatus; Paradonus gallatinensis, Paradonus gustafsoni, Paradonus stibicki; Siamites langkatensis, S. thantonus, S. sumatrensis, S. sabahensis; Sathytes communis, S. fenghuang, S. jinggang, S. obliquus, S. similis; Parygrus guarani, P. lengua, P. maya, P. quechua, P. zamuco; Pseudastenus amazonicus, P. ferrugineus, P. latus, P. oculatus, P, ribeirocostae, P. schubarti; Nazeris mahuanggouensis, Nazeris zhouhaishengi, Nazeris wuluozhenensis; Galerucella anserina; Oemina Gen. sp.; Anthobiomorphus rougemonti, A. makranczyi; Nesanoplium spinosum, Psyrassa zacki, Euderces ecuadorensis, Xela tysoni; Glaresis gentile; Physoglossus devagiriensis; Trigonotoma digitata, T. constricta; Exocelina sugayai; Acroleptus alvarengai, Acroleptus limai, Aporrhipis obrieni, Aporrhipis milleri; Novius marek; Analophus septentrionalis, Archetypus marginatus, Brephilydia fearni, Eurynassa tuberculicollis, Hagrides mandibularis, Hermerius occidentalis;
- 1 new neuropteran: Coniopteryx (Scotoconiopteryx) burmeisteri;
- 44 new hymenopterans: Aulacidea turcica; Gnamptogenys lenis, G. latistriata, G. avus; Allodynerus reduncus, Allodynerus bimaculus, Allodynerus diqingensis, Allodynerus asperipunctarus; Dipogon (Stigmatodipogon) chiangmai, D. (S.) himalayensis; Xiphydria kanba, X. konishii, X. melanoptera; Eremotylus pukayana; Xorides tertiusdecimus; Jezonogonalos nyingchiensis, Taeniogonalos eurysoma; Aleiodes carbonaroides, A. coriaceus, A. improvisus, A. nigrifemur, A. turcicus, A. zwakhalsi; Nylanderia deyrupi, Nylanderia parasitica; Isepeolus mankalunthata, Melectoides licancabu, M. desiccata, M. glaucodontus; Torymus achyranthii, T. aciculatus, T. acutissimus, T. angustitemple, T. brevicaudatus, T. hirtipennis, T. macrops, T. matsunagae, T. minamii, T. quernus, T. rugosus, T. sasae, T. sawadai, T. wanggyui; Nemeritis ananenkoi, N. baranovi, N. bespalovi, N. legasovi;
- 53 new dipterans: Ins pectorcolumbo, Ins zanouts; Cordilura katoi, C. shinonagai, C. yezoana; Chvalaea yolkamini; Antennardia suorkensis, Aprionus mossbergi, Apr. oljonsbynensis, Ladopyris baltica gen. et sp. nov. (found also in Estonia), Monardia (M.) lapponica, Monardia (Xylopriona) abbreviata, Mon. (Xyl.) obscura, Neurolyga simillima, N. taigensis sp. nov. (found also in the Republic of Karelia, Russian Federation), Peromyia elongatula, P. lindstroemi, P. sofielundensis; Myolepta angustifacia; Systellapha digifurca, S. ornatifemur; Pseudolycoriella angustoantennata, Psl. breviradiata, Psl. consectaria, Psl. fuscovenosa, Psl. globostylata, Psl. notanda, Psl. paucispinata, Psl. secura, Psl. unispinata; Pagastia (P.) donoliveri; Silba mitsuii, Silba fungicola; Chaetonerius stichodactylus; Cryptocladocera arnaudi; Asphondylia singanallurensis; Triphleba simovi; Simulium itajara; Forcipomyia (F.) pyrenaica, Monohelea mediterranea; Megaselia aliusalius, M. exwignatpark, M. pilusdepilata, M. polonici, M. quasirufifrons, M. reduncus, M. setaeneclobi; Hemerodromia aliaextriata, H. deprimatura, H. oretenebraea, H. pairoti, H. samoha;
- 5 new trichopterans: Chimarra gangtokensis; Stactibiella aichi, S. amami, S. kumejima; Acostatrichia araca;
- 36 new lepidopterans: Promalactis costispinata, P. trimaculata, P. simingshana, P. yongjiana; Promalactis clavivalvata, P. abasiloba, P. trusmadiensis; Promalactis unidentalis, P. tenuiclavifera, P. introflexa, P. bolikhamsaiana, P. serrulata; Nerice (Nerice) mishmiensis; Grapholita thermopsidis; Evonima tianmuensis; Victrix svetlanae; Alychna chulumani, Alychna argenteus; Diduga luteogibbosa, D. allodubatolovi, D. scalprata, D. hainanensis; Stenoloba herbacea, Stenoloba pontezi; Gymelloxes juliusboosi; Calonola theresiae; Odonestis bolotovi; Eupterote elisavetae; Phaegoptera touroulti, Phaegoptera doroshkini; Vietomartyria wuyunjiena, V. maoershana; Lasiochira wuzhishanensis; Chrysorthenches muraseae, C. smaragdina; Mathania hughesi;

Echinoderms
- 5 new asteroids: Lophaster cactorum, Paralophaster gomo, Hyalinothrix diversus, Hyaliothrix enoki, Hyalinothrix virtrispinum;
- 2 new holothuroids: Thyone brasiliana, Havelockia nietae;

Actinopterygians
- 1 new anguilliform: Ophichthus olivaceus;
- 1 new blenniiform: Cirripectes matatakaro;
- 4 new characiforms: Knodus cupariensis; Hemiodus tucupi; Creagrutus sp.; Hyphessobrycon chiribiquete;
- 1 new clupeiform: Encrasicholina sigma;
- 4 new cypriniforms: Garra barreimiae, Garra gallagheri; Parapsilorhynchus alluriensis; Psilorhynchus kamengensis;
- 2 new gadiforms: Euclichthys microdorsalis, E. robertsi;
- 1 new gobiiform: Silhouettea ghazalae;
- 2 new perciforms: Plectranthias cruentus; Verilus costai;

Amphibians
- 15 new anurans: Eleutherodactylus erythrochomus; Pristimantis ardilae, Pristimantis bowara; Pristimantis giorgii, Pristimantis pictus, Pristimantis pluvian, Pristimantis moa; Hyloxalus arliensis; Allobates pacaas; Bufo (Anaxyrus) nevadensis, Bufo (Anaxyrus) monfontanus; Phrynobatrachus arcanus, P. mbabo; Boana nigra, B. ventrimaculata;
- 2 new caudates: Necturus mounti, N. moleri;

Reptiles
- 12 new squamates: Panaspis mocamedensis; Acanthodactylus lacrymae, A. montanus; Dryocalamus chithrasekarai; Cyrtodactylus evanquahi; Xylophis mosaicus; Hemiphyllodactylus ngwelwini, H. kyaiktiyoensis, H. pinlaungensis, H. zwegabinensis; Emydocephalus orarius; Smaug swazicus;
– – –
– – –
* This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.