Intestinal parasites infecting captive non-human primates in Italy
- 1Department of Public Health and Infectious Diseases, Sapienza University of Rome, Rome, Italy
- 2Department of Food Safety, Nutrition and Veterinary Public Health, Istituto Superiore di Sanità, Rome, Italy
- 3Istituto Zooprofilattico Sperimentale del Lazio e della Toscana “Mariano Aleandri”, Rome, Italy
- 4Department of Clinical Sciences and Translational Medicine, University of Rome Tor Vergata, Rome, Italy
- 5Parco Faunistico Piano dell’Abatino, Rieti, Italy
Non-human primates (NHPs) living in captive conditions are susceptible to intestinal parasites that can contribute to mortality and morbidity, and cause zoonotic infections. Thus, parasite surveys on NHP populations under human care are relevant as part of the evaluation of NHPs welfare and in the zoonotic disease risk assessment, as well as in the exploration of parasite transmission pathways, according to the One-Health concept. This study aimed to identify intestinal parasites infecting NHPs living in two wildlife recovery centers and in a zoological garden, in Italy. Ninety-three fecal samples from Macaca tonkeana, Macaca fascicularis, Sapajus apella, Chlorocebus aethiops, Macaca fuscata, Macaca sylvanus, and Cebus capucinus were collected at Piano dell’Abatino Park (Lazio), and fecal smears and flotation were performed in order to identify parasites according to morphological keys. Additionally, one carcass of M. fuscata from the Bioparco Zoological Garden of Rome (Lazio) and one of M. fascicularis from the Center for the Recovery of Exotic and Maremma Wild Animals (Tuscany) were necropsied and intestinal adult nematodes were collected and characterized at morphological and molecular level, using the mitochondrial cox1 and rrnL markers. Protozoans (Entamoeba coli, Iodamoeba bütschlii, Dientamoeba fragilis-like, Giardia sp.), chromists (Balantidium/Buxtonella sp.) and nematodes (Capillaria sp., Trichuris sp., strongyliform larvae and Oesophagostomum sp.) were found through fecal smears and flotation. The collected adult nematodes from dead NHPs were morphologically identified as whipworms (genus Trichuris). Phylogenetic analyses grouped Trichuris specimens into the Trichuris trichiura complex of species, with specimens from M. fuscata clustering into a host-specific branch, and whipworms from M. fascicularis clustering within a clade formed by Trichuris infecting several primate species, including humans. The results here collected revealed the presence of potentially zoonotic parasites circulating in captive primates in Italy, providing useful information for the formulation of management and care plans for captive NHPs, and for the elaboration of safety measures for visitors and animal keepers.
1 Introduction
Intestinal parasites are often responsible for diseases in animals living in confined environments such as sanctuaries, zoological gardens and wildlife rescue centers (1). Captive animals may be more susceptible to protozoan and helminth parasites with direct life cycles, which are more prevalent and prone to disseminate in confined conditions where the animals might be more stressed due to overpopulation and malnutrition, showing clinical signs as diarrhea and dehydration, and requiring veterinary care (2, 3). Parasite transmission mainly occurs through the fecal-oral route via direct contact with infected hosts (or their fecal material), or indirectly through the ingestion of contaminated water or food (4). In a confined environment, the low hygienic measures may lead to high levels of environmental contamination, and the handlers’ movements among different premises without safe and hygiene measures may contribute to the dissemination of such parasites inside and outside the workplace. Captive non-human primates (NHPs) may act as reservoirs for zoonotic parasites and the frequent use of pharmacological treatments may lead to the selection of resistance traits (1, 5). Therefore, confined environments are of great interest for parasitological studies, involving the One-Health concept.
Parasitological investigations have been carried out worldwide in zoological parks housing NHPs. For instance, Giardia duodenalis infections were reported in several NHPs hosted in 12 zoological gardens in China (6), while in a study carried out in Malaysia in three zoos hosting 69 specimens of NHPs, there were reported 21 species of intestinal parasites with a high prevalence of nematodes like Ascaris spp. and Oesophagostomum spp., only one animal positive to Blastocystis and no observation of Giardia spp. (7). Moreover, a large survey on intestinal parasites infecting NHPs hosted in two research centers in Brazil reported a large occurrence of Balantidium coli and Entamoeba sp. among protozoans, and a general low frequency of helminths, with predominance of Trichuris trichiura (8).
In Europe, parasitological surveys on NHPs have been performed in zoological enclosures such as the Dublin Zoological Garden (Ireland) (9), the Belgrade Zoo (Serbia) (10), the Kiev Zoo (Ukraine) (11), the Brno Zoological Garden (Czech Republic) (12), the Sofia Zoo (Bulgaria) (13), the Wroclaw Zoo (Poland) (14), the Košice Zoological Garden (Slovakia) (15), among others (13, 16). Nematodes (e.g., Ascaris sp., Trichuris sp., Strongyloides sp.) are the most common parasites detected, followed by cestodes and trematodes (13). Furthermore, G. duodenalis, Cryptosporidium hominis, Blastocystis sp., and Entamoeba dispar circulation between NHPs and their zookeepers has been identified in European zoological gardens, with the confirmation of zoonotic transmission events involving Blastocystis sp. and a highly suspected zoonotic transmission of C. hominis (4). Additionally, subcutaneous Taenia crassiceps cysticercosis in a ring-tailed lemur in a Serbian zoo has been reported (17).
In Italy, some surveys on intestinal parasites infecting NHPs living in zoological gardens have been conducted so far. In central Italy, Cryptosporidium sp. and Trichuris sp. have been found infecting Lemur catta at the Giardino Zoologico of Pistoia (18), while, at the Bioparco Zoological Garden of Rome, G. duodenalis has been reported infecting L. catta, and Entamoeba spp. was diagnosed in Cercocebus torquatus, Chlorocebus aethiops, Macaca fuscata, Mandrillus sphinx, Pan troglodytes, L. catta, and Pongo pygmaeus (19). In southern Italy, Trichuris sp., Strongyloides fuelleborni, and Cryptosporidium sp. infected Papio cynocephalus at the Fasano Zoo Safari, while G. duodenalis was found infecting L. catta, Cercopithecus mona, Alouatta caraya, Nomascus concolor, Colobus guereza, and Semnopithecus entellus in a zoological garden in the Benevento province (20). Moreover, Cyclospora was detected in P. troglodytes from a wildlife animal rescue center, and in Macaca fascicularis from an experimental primate research center (21). Eight taxa of intestinal parasites (Trichuris sp., Oesophagostomum sp., Entamoeba coli, Endolimax nana, Iodamoeba bütschlii, Chilomastix mesnili, B. coli, and Blastocystis sp.) were recorded infecting M. fascicularis in a biomedical research center (22).
Concerning necropsies carried out on dead captive NHPs, Trichuris sp. from Eulemur albifrons, and Strongyloides sp. from Macaca sylvanus have been found at the Natura Viva zoo (23), and Echinococcus granulosus from L. catta at a zoo in northern Italy (24). At the Bioparco Zoological Garden of Rome, larval forms of Taenia martis from L. catta (25), and adult Trichuris sp. from L. catta, M. fuscata and C. aethiops have been reported (26, 27). Larval forms of Mesocestoides sp. from Saguinus midas were collected at a wildlife recovery center (28).
Despite their importance in public health and NHPs welfare, the currently available information on intestinal parasites infecting captive NHPs in Italy is still limited to fragmented data. Thus, here we provide a survey on intestinal parasites circulating in NHPs hosted in two wildlife rescue centers and in one zoological garden in central Italy.
2 Materials and methods
Fecal samples and adult nematodes were collected during 2020–2022, from NHPs living in confined environments in Italy.
2.1 Fecal samples
Ninety-three fecal samples from Macaca tonkeana (Tonkean black macaque) (n = 23), Macaca fascicularis (long-tailed macaque) (n = 16), Sapajus apella (tufted capuchin) (n = 43), Macaca fuscata (Japanese macaque) (n = 2), Macaca sylvanus (Barbary macaque) (n = 4), Chlorocebus aethiops (grivet) (n = 2) and Cebus capucinus (capuchin monkey) (n = 3) were collected at the Piano dell’Abatino Park (Lazio), in the framework of a routine parasitological survey. In this habitat, the animals are hosted in different premises, as detailed below. One premise hosts two individuals of C. aethiops and two individuals of C. capucinus; one premise is dedicated to M. fascicularis, with seven individuals; two not-separated premises for M. sylvanus with 11 individuals; one premise for only one individual of M. fuscata; three premises for S. apella, with 11, 12, and 14 individuals; and four premises for M. tonkeana with eight, six, thirteen, and fourteen individuals. Fresh samples were collected directly from the soil inside the premises and were not attributed to a specific individual. For each sample, one aliquot was stored in 10% formalin solution, and one aliquot in 70% ethanol solution. Samples were examined both macroscopically, to verify the presence of nematodes or cestodes, and microscopically. Morphological identification of protozoan and helminth parasites was performed after direct fecal smears (29) and flotation with a salt-sugar solution (SG: 1.28) (30) useful for general purposes. Slides from direct fecal smears and flotation were examined with a microscope, and at least 10 fields were screened at objective magnification ×100, ×200, ×400, and ×1,000, successively. This protocol was used to qualitatively identify parasite eggs, cysts and oocysts. Photos of parasites were taken for morphological identification. For some parasite taxa the identification was possible only to the genus level.
2.2 Adult nematodes
Two dead macaques were inspected during necropsies carried out at the Istituto Zooprofilattico Sperimentale del Lazio e della Toscana “Mariano Aleandri” to identify the cause of death. Ten entire adult nematodes (three males and seven females) and few disrupted nematode body portions were collected from the caecum of one M. fascicularis hosted at the Center for the Recovery of Exotic and Maremma Wild Animals (CREMWA) (Tuscany). From one M. fuscata hosted at the Bioparco Zoological Garden of Rome (Lazio), eight adult nematodes (all females - not well preserved) were collected from the caecum. Nematodes were repeatedly washed with saline solution, and then used for morphological observation after clarification in lactophenol. A body portion was used for molecular characterization based on sequence analyses of the two partial mitochondrial regions cox1 and rrnL, informative for phylogenetic assignment (31, 32). The obtained sequences were compared to homologous GenBank retrieved data, and used for phylogenetic inferences with the maximum likelihood (ML) method by MEGA7 (33), after testing for the best evolutionary models explaining the data (33). The only available homologous sequences of Trichuris sp. from the same host species M. fascicularis (JF690967) was not reliably attributable to this genus, thus it was excluded from the analysis. Sequences of Trichinella spiralis and Trichinella britovi were used as outgroups (AF293969, KM357413). Additional file 1 and file 2 show the material used for comparative analyses.
3 Results
3.1 Fecal samples
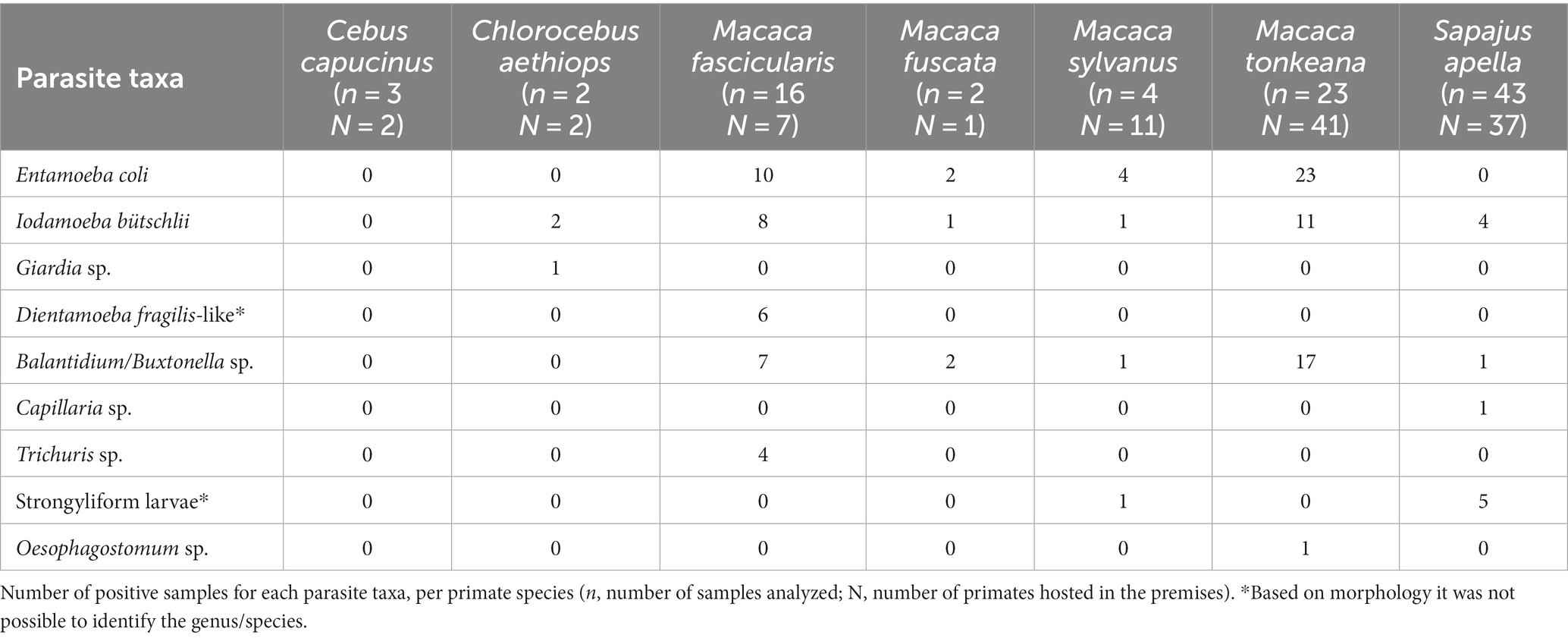
Four taxa of protozoans (Entamoeba coli, Iodamoeba bütschlii, Dientamoeba fragilis-like, and Giardia sp.), one taxon of chromist (Balantidium/Buxtonella sp.), and four taxa of helminths (Capillaria sp., Trichuris sp., strongyliform larvae and Oesophagostomum sp.) were identified in the fecal samples from NHPs living at the Piano dell’Abatino Park (Table 1). None infected animals showed gastrointestinal symptoms. Representative images from microscopic analyses are available in the Figure 1.
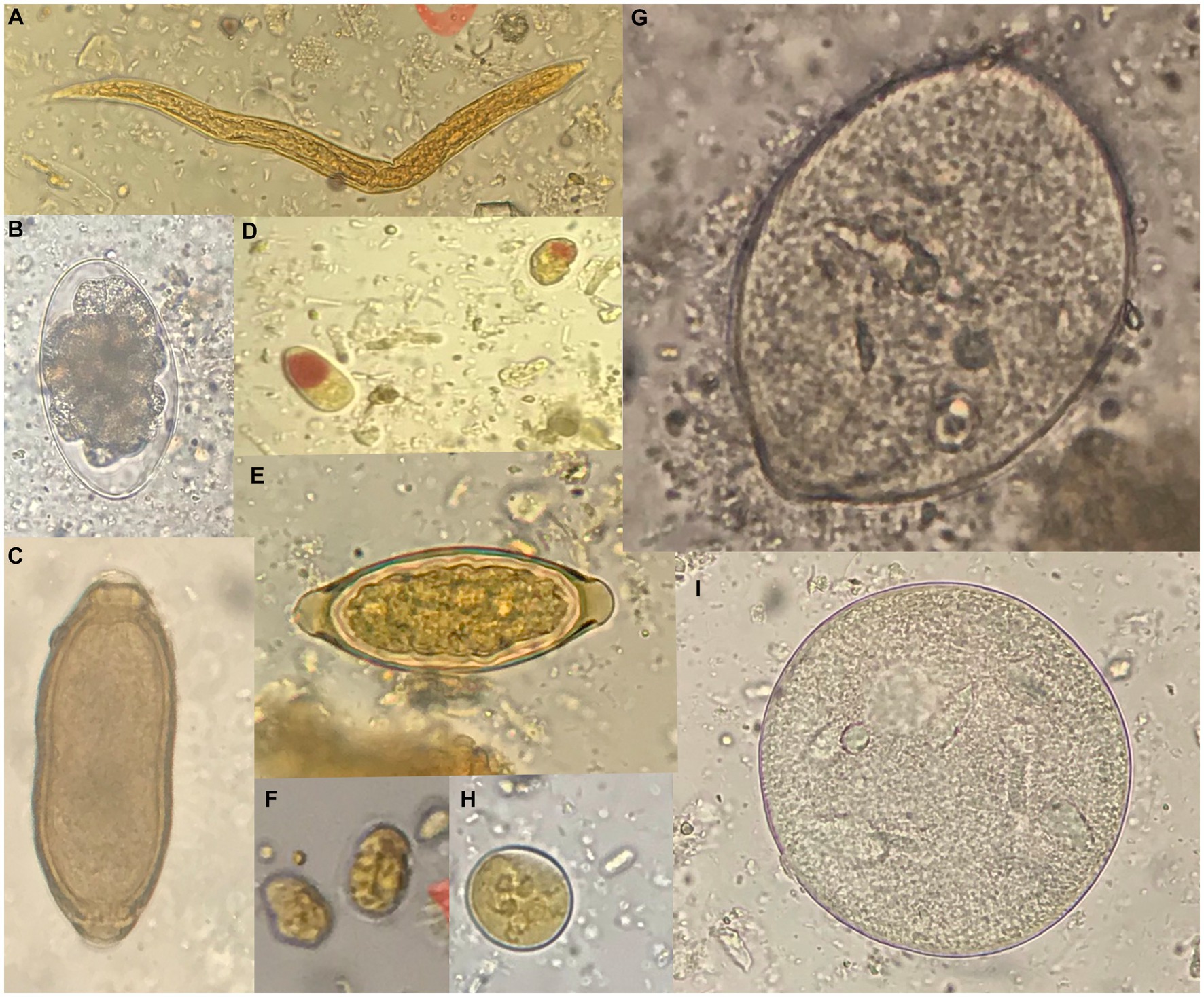
Figure 1. Representative images of parasites detected by microscopy. (A) Strongyliform larva (40x). (B) Oesophagostomum sp. (50 × 85 μm). (C) Capillaria sp. (40 × 25 μm). (D) Iodamoeba bütschlii (10 × 12 μm). (E) Trichuris sp. (25 × 55 μm). (F) Giardia sp. (10 × 8 μm). (G) Balantidium/Buxtonella sp. trophozoite (90 μm). (H) Entamoeba coli (20 μm). (I) Balantidium/Buxtonella sp. cyst. Measures refer to the samples shown in the figure.
The capuchin monkeys were the only primate species in which no gastrointestinal parasites were observed. The following potentially zoonotic parasites were detected: Giardia sp. was found infecting the grivet, Trichuris sp. infecting the long-tailed macaque, Oesophagostomum sp. was observed in the Tonkean black macaque and Capillaria sp. in the tufted capuchin monkey. Trichuris sp. and Capillaria sp. were not identified at species level due to negative results of molecular identification assays.
3.2 Adult nematodes
The general gross morphology of Trichuris adult specimens collected from M. fascicularis and M. fuscata intestinal caeca was congruent with a filiform long anterior part and a broad and handle-like posterior part, typical of whipworms. The cuticle presented transversal striation and the anterior portion of the body showed bacillary bands. Males (Figure 2) and females (Figure 3) showed similar morphological features described for Trichuris trichiura from Papio papio and M. sylvanus (31), Trichuris sp. from M. sylvanus (34, 35) and T. ursinus from Papio ursinus (36). The eggs measurements ranged from 25.50–27.90 × 54.30–56.80 μm in Trichuris from M. fascicularis and from 30–35 × 53–61.6 μm in Trichuris from M. fuscata.
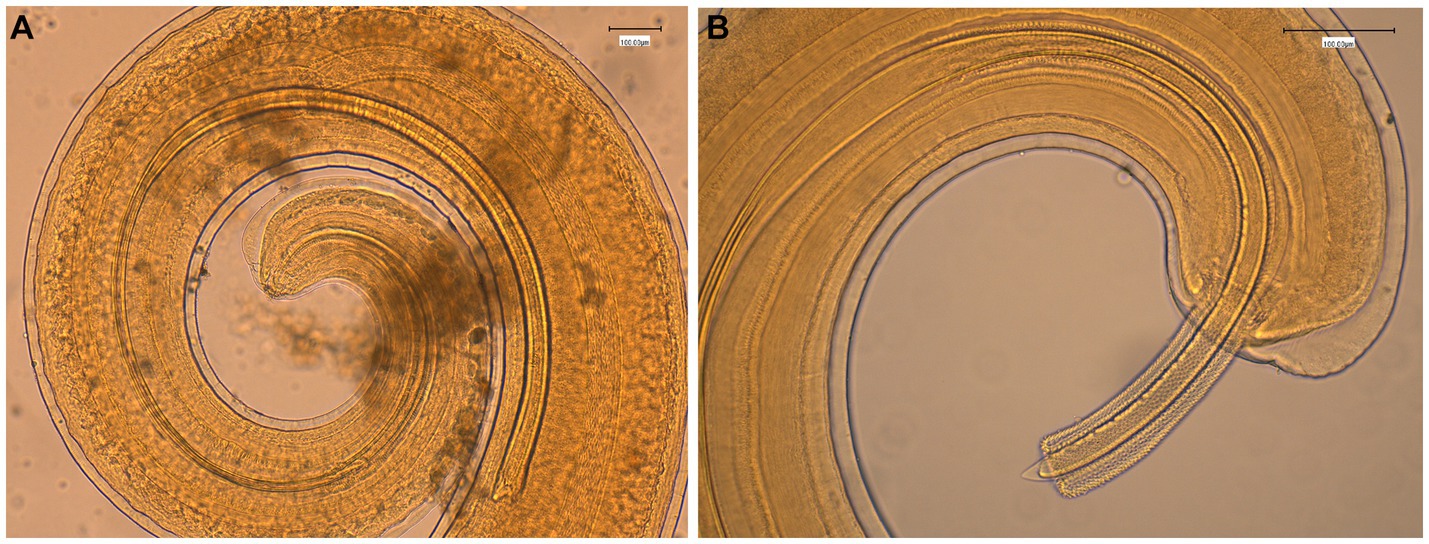
Figure 2. Morphology of male Trichuris sp. from Macaca fascicularis. (A) Posterior end showing the arrowed and invaginated spicule, with distal and proximal cloacal tube and ejaculatory duct. (B) Posterior end with evaginated spicule and spicule sheath with spines.
Figure 3. Morphology of female Trichuris sp. from Macaca fascicularis. (A) Vulva region with visible tegument covered by spines. (B) Circumvoluted vagina with eggs. (C) Posterior end showing the end of uterus and cloaca.
Regarding the molecular characterization, ten high quality rrnL sequences (nine from M. fascicularis and one from M. fuscata) and four cox1 sequences (all from M. fascicularis) were obtained from the collected nematodes and used for phylogenetic inferences in comparison to GenBank retrieved data, with final datasets of 43 input and 460 bp and of 32 input and 341 bp, respectively. Both phylogenetic trees identified the presence of two main clades, namely “Clade 1” and “Clade 2” (31). The rrnL ML consensus tree in Figure 4 described Clade 1 named as the T. suis clade, including Trichuris colobae as a sister clade of T. suis + Trichuris sp. from Chlorocebus. The Trichuris specimens from M. fascicularis here analyzed clustered into the “subclade c” of the “Clade 2” or T. trichiura clade branch (indicated in red color) (31), with high statistical support (99–100%). The “subclade c” branch included T. trichiura individuals collected in a broad host range for primates, such as the Japanese macaque, the Barbary macaque, the green monkey, the baboon, and humans from Africa and Europe. The specimen from M. fuscata here collected grouped in the subclade defined as MF in previous reports (branch indicated in blue color) from the same host species living in the Bioparco Zoological Garden of Rome (26).
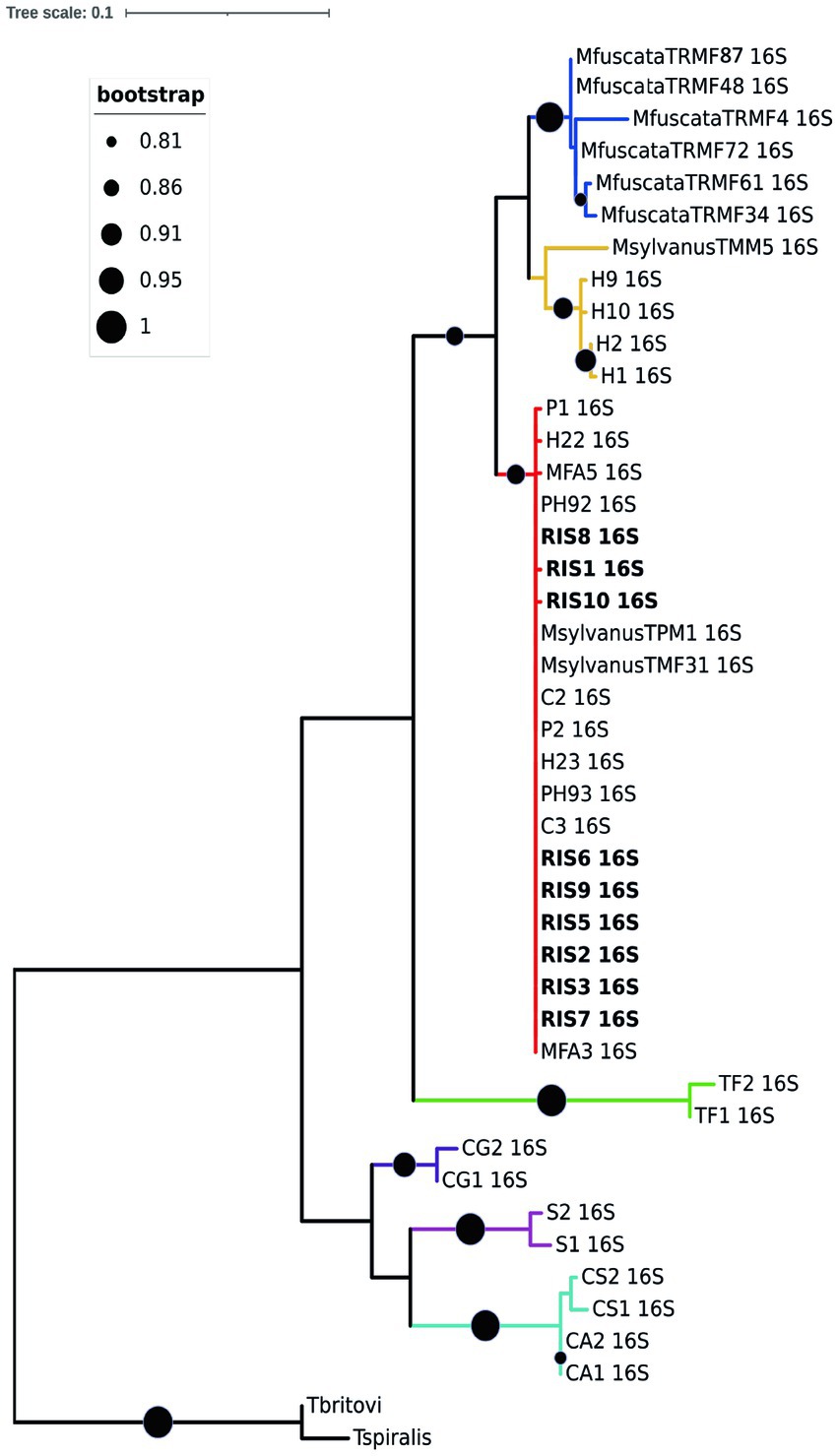
Figure 4. Phylogenetic tree based on rrnL of Trichuris spp. Maximum likelihood consensus tree of partial mitochondrial rrnL of Trichuris spp. analyzed in the present study (for specimen codes information see Supplementary File S1). Circles at nodes indicate the bootstrap statistical support, according to the legend on the left. Colors represent clades and sub-clades and correspond to those reported by Rivero et al. (31).
A similar topology was obtained for the cox1 ML consensus tree (Additional file 3), in which specimens of Trichuris from M. fascicularis were included in the “Clade 2 subclade c” together with Trichuris from other macaques and baboons. Such evidences confirmed that specimens infecting M. fascicularis here analyzed can be identified as T. trichiura, given the similarity with this taxon reported also in other primates, including humans. No good quality sequences were obtained at this marker for Trichuris infecting M. fuscata.
4 Discussion
The present study investigated the presence of intestinal parasites infecting NHP species living in captivity in Italy. Based on morphological analyses from fecal samples of NHPs living at Piano dell’Abatino Park, nine parasite taxa were identified, all of them presenting direct life cycles. However, due to the sampling from premises with multiple hosts, without tracing primate individuals during defecation, the precise estimation of epizootiological parameters such as prevalence, intensity and abundance was not possible.
In this study, Balantidium/Buxtonella sp., E. coli, and I. bütschlii were the most frequently detected parasites. Most parasite taxa identified in this study have been previously reported in captive NHPs in Europe, as is the case of Trichuris sp., Oesophagostomum sp., Balantidium sp., Giardia sp., E. coli and I. bütschlii (19, 22). Giardia sp. was found in only one individual, and taking into account that this parasite is usually more frequently found in studies on NHPs in zoos, we should consider that in this case it may not be a true infection but cysts accidentally ingested from the environment. Additionally, due to the intermittent shedding of cysts, in some cases it is necessary the examination of fecal samples on consecutive days (47), and in this study no sampling on consecutive days was performed.
Balantidium/Buxtonella sp. was found infecting five of the seven NHP species sampled. Pigs are the main reservoir host of Balantidium, while rodents and NHPs may function as alternative reservoir hosts (37). Wild boars are also present at the study site, but in a small number and located in a separate facility from the NHPs. Thus, in this case swine are unlikely to participate in the transmission cycle (even if it cannot be definitively ruled out due to handler’s movements, or by the rain/wind that can easily transport the cysts from one facility to another), while wild rodents are very common within the primate enclosures. For future studies it is highly recommended the use of integrative taxonomy accounting for morphological characteristics combined with molecular approach for species identification, as it has been demonstrated how misleading the cyst morphology-based diagnostics of Balantidium sp. and Buxtonella sp. can be, leading to ambiguity in the epidemiology of these infections (38). In Italy, both Buxtonella sp. and Balantidium sp. have been reported, for instance Buxtonella sulcata infecting cattle in central Italy (39), and Balantidium coli infecting swine in the south of the country (40). Given the uncertainty in the taxonomic assignment, we have chosen to indicate this finding as Balantidium/Buxtonella sp.
Molecular testing should be also recommended for the optimal identification of D. fragilis (41). In our survey, D. fragilis-like was found in samples from M. fascicularis, and this parasite was recently reported infecting free-ranging M. fascicularis in Indonesia (42). Additionally, future molecular studies to determine the species of the strongyliform larvae found infecting S. apella and M. sylvanus are required, in particular to confirm or exclude the presence of Strongyloides sp., a zoonotic parasite of paramount relevance, reported in Italy both in dogs and humans (43). Capillaria sp. was found in one sample of a tufted capuchin monkey, however, the molecular approach for species identification gave negative results, probably due to difficulties in the genomic DNA isolation and/or PCR inhibitors. Capillaria sp. has been reported infecting different NHP species (44), including capuchin monkeys: C. capucinus in Panama (45) and C. albifrons in Ecuador (46). However, these reports were based on microscopy, thus, the use of molecular testing is also here suggested for the identification at species level to elucidate the zoonotic potential.
Trematodes, cestodes and acanthocephalans have been previously reported in free-ranging primates (48). Considering that the methods used in this study allow the detection of these parasite taxa, the lack of findings could be related to the different diets and habits of captive individuals compared to free-ranging NHPs.
Given the close phylogenetic relationship between human and NHPs, continuous parasitological surveys on captive primates should be encouraged for the monitoring of zoonotically transmitted parasites, for instance within conservation and management of threatened primate species, and in the recovery of traded NHPs. In the present study, four out of the seven NHP species under investigation are considered endangered (EN) or vulnerable (VU) by the IUCN, and while no animals hosted at Piano dell’Abatino Park showed clinical signs or symptoms of gastrointestinal origin, two animals died at the Bioparco Zoological Garden of Rome and in the CREMWA, probably because of Trichuris infection. So far, Trichuris spp. have been reported by morphological and/or molecular characterization in the following Macaca species: the Japanese macaque (26, 27, 32), the Barbary macaque (34, 35) and the long tailed macaque (22), the latter investigated only in terms of eggs presence in stool samples without any molecular characterization. Such studies revealed the presence of two separated taxonomic entities able to infect Japanese macaques living in confined environments, one specific to this host, and one shared also with other primates (26, 27, 32). Analogous molecular results were obtained also regarding the Barbary macaque hosted in the Castellar Zoo (Spain), infected by two genotypes within the T. trichiura lineage, supported also by morphological data (35).
Here we provide for the first time morphological and molecular data of T. trichiura infecting M. fascicularis, to share with the scientific community for comparative purposes. We obtained reliable data from the analyses of adult Trichuris infecting the dead long tailed macaques hosted at the CREMWA, and despite no molecular data were obtained from fecal samples from the animals hosted at Piano dell’Abatino Park, the eggs size observed in the two sample sites were overlapping, suggesting T. trichiura circulation. The long-tailed macaque from the CREMWA analyzed in the present study lived in a colony of around 30 individuals (49), thus the finding of Trichuris infection may represent a high risk for the other macaques belonging to the colony. It is also a concern, taking into account that M. fascicularis has been recently listed as an endangered species with a decreasing population trend, according to the International Union for Conservation of Nature (IUCN) (50), mainly due to the high demand in the national and international trade, and the hunting for subsistence. Moreover, there is a risk for handlers and visitors in terms of zoonotic transmission. Therefore, it is necessary the constant monitoring to trace the presence of eventual parasitic species of zoonotic interest, in both confined environments and in native areas where NHPs live near or in close contact with humans.
In conclusion, this parasitological survey revealed the presence of potentially zoonotic parasites circulating in NHPs in Italy, providing useful information for the formulation of their management and care plans, and for the elaboration of safety measures for visitors and animal keepers. Regular parasitological surveys in captive NHPs using both microscopy and molecular analyses should be recommended, in order to monitor the impact of parasitosis on the health status of captive NHPs and to properly assess the potential zoonotic transmission risk.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because Biological material taken from alive animals was not invasively collected, while material taken during necropsies was authorized by the ethical approval of the Istituto Zooprofilattico Sperimentale Lazio e Toscana.
Author contributions
SR: Conceptualization, Funding acquisition, Methodology, Writing – original draft. SC: Conceptualization, Formal analysis, Methodology, Writing – original draft. MMDF: Formal analysis, Writing – review & editing. CD: Resources, Writing – review & editing. FB: Supervision, Writing – review & editing. NC: Resources, Writing – review & editing. SD’A: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research received funding from Sapienza University of Rome trough the “Avvio alla Ricerca Tipo 2” (Protocol number: AR2221811FCCAA02) awarded to SR.
Acknowledgments
Authors thank Antonio De Marco, Laura Toti, Denise De Martino, and Elena Pirri for providing all logistic support at Piano dell’Abatino Park. We thank Ilaria Bellini, Claudia Chiovoloni, Amata Petracca, and Antonella Pizzarelli for the support in the collection of fecal samples.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1270202/full#supplementary-material
Supplementary Material 1 | Trichuris sp. material used for phylogenetic inference based on the partial mitochondrial ribosomal rrnL region. Information on specimen codes, host species, GenBank accession number and literature references are provided.
Supplementary Material 2 | Trichuris sp. material used for phylogenetic inference based on the partial mitochondrial cox1 region. Information on specimen codes, host species, GenBank accession number and literature references are provided.
Supplementary Material 3 | Maximum likelihood consensus tree of the Trichuris spp. partial mitochondrial cox1 sequences analyzed in the present study. Numbers at nodes indicate the bootstrap statistical support (for specimen codes information see Additional file 2).
References
1. Kvapil, P, Kastelic, M, Dovc, A, Bártová, E, Cížek, P, Lima, N, et al. An eight-year survey of the intestinal parasites of carnivores, hoofed mammals, primates, ratites and reptiles in the Ljubljana zoo in Slovenia. Folia Parasitol (Praha). (2017) 64:1–6. doi: 10.14411/fp.2017.013
2. Vonfeld, I, Prenant, T, Polack, B, Guillot, J, and Quintard, B. Gastrointestinal parasites in non-human primates in zoological institutions in France. Parasite. (2022) 29:43. doi: 10.1051/parasite/2022040
3. Levecke, B, Dorny, P, Geurden, T, Vercammen, F, and Vercruysse, J. Gastrointestinal protozoa in non-human primates of four zoological gardens in Belgium. Vet Parasitol. (2007) 148:236–46. doi: 10.1016/j.vetpar.2007.06.020
4. Köster, PC, Martínez-Nevado, E, González, A, Abelló-Poveda, MT, Fernández-Bellon, H, de la Riva-Fraga, M, et al. Intestinal protists in captive non-human primates and their handlers in six European zoological gardens. Molecular evidence of zoonotic transmission. Front Vet Sci. (2022) 8:819887. doi: 10.3389/fvets.2021.819887
5. Mir, AQ, Dua, K, Singla, LD, Sharma, S, and Singh, MP. Prevalence of parasitic infection in captive wild animals in Bir Moti Bagh mini zoo (deer park), Patiala, Punjab. Vet World. (2016) 9:540–3. doi: 10.14202/vetworld.2016.540-543
6. Zhang, X, Wang, L, Lan, X, Dan, J, Ren, Z, Cao, S, et al. Occurrence and multilocus genotyping of Giardia duodenalis in captive non-human primates from 12 zoos in China. PLoS One. (2020) 15:e0228673–11. doi: 10.1371/journal.pone.0228673
7. Adrus, M, Zainudin, R, Ahamad, M, Jayasilan, M, and Abdullah, M. Gastrointestinal parasites of zoonotic importance observed in the wild, urban, and captive populations of non-human primates in Malaysia. J Med Primatol. (2019) 48:22–31. doi: 10.1111/jmp.12389
8. da Silva, BA, Pissinatti, A, Dib, L, de Siqueira, M, Cardozo, M, Fonseca, A, et al. Balantidium coli and other gastrointestinal parasites in captives non-human primates of the Rio de Janeiro, Brazil. J Med Primatol. (2015) 44:18–26. doi: 10.1111/jmp.12140
9. Geraghty, V, Mooney, J, and Pike, K. A study of parasitic infections in mammals and birds at the Dublin zoological gardens. Vet Res Commun. (1982) 5:343–8. doi: 10.1007/BF02215003
10. Nesic, D, Pavlovic, I, Valter, D, Savin, Z, and Hudina, V. Endoparasite fauna of primates at Belgrade zoo. Vet Glas. (1991) 42:365–7.
11. Kharchenko, V, and Marunchin, A. Helminths from the mammals of the Kiev zoo. Vestn Zool. (1992) 3:61–3.
12. Hartmanova, B, Hojovcova, M, and Fiala, L. Parasitic diseases of monkeys and large feline predators in the zoological garden in Brno. Sb Ved Pr Ustred Statniho Vet Ust. (1987) 17:44–5.
13. Panayotova-Pencheva, MS . Parasites in captive animals: a review of studies in some European zoos. Zool Garten. (2013) 82:60–71. doi: 10.1016/j.zoolgart.2013.04.005
14. Oculewich, A, and Kruczkowska, B. Intestinal parasites of monkeys in the Wroclaw zoo. Iad Parazytol. (1988) 34:301–5.
15. Danišová, O, Valenčáková, A, Kandráčová, P, Tomko, M, and Sučik, M. First report of Blastocystis spp. subtypes in ZOO animals in Slovakia, Central Europe. Ann Agric Environ Med. (2022) 29:149–51. doi: 10.26444/aaem/145826
16. Nosková, E, Modrý, D, Baláž, V, Červená, B, Jirků-Pomajbíková, K, Zechmeisterová, K, et al. Identification of potentially zoonotic parasites in captive orangutans and semi-captive mandrills: phylogeny and morphological comparison. Am J Primatol. (2023) 85:e23475. doi: 10.1002/ajp.23475
17. Simin, S, Vračar, V, Kozoderović, G, Stevanov, S, Alić, A, Lalošević, D, et al. Subcutaneous Taenia crassiceps cysticercosis in a ring-tailed lemur (Lemur catta) in a Serbian zoo. Acta Parasitol. (2023) 68:468–72. doi: 10.1007/s11686-023-00679-w
18. Fagiolini, M, Lia, R, Laricchiuta, P, Cavicchio, P, Mannella, R, Cafarchia, C, et al. Gastrointestinal parasites in mammals of two Italian zoological gardens. J Zoo Wildl Med. (2010) 41:662–70. doi: 10.1638/2010-0049.1
19. Berrilli, F, Prisco, C, Friedrich, KG, Di, CP, Di, CD, and De, LC. Giardia duodenalis assemblages and Entamoeba species infecting non-human primates in an Italian zoological garden: zoonotic potential and management traits. Parasit Vectors. (2011) 4:199. doi: 10.1186/1756-3305-4-199
20. Capasso, M, Ciuca, L, Procesi, IG, Zinno, F, Berrilli, F, Cringoli, G, et al. Single and synergistic effects of fenbendazole and metronidazole against subclinical infection by Giardia duodenalis in non-human primates in a zoological garden in southern Italy. Front Vet Sci. (2022) 9:929443. doi: 10.3389/fvets.2022.929443
21. Marangi, M, Koehler, AV, Zanzani, SA, Manfredi, MT, Brianti, E, Giangaspero, A, et al. Detection of Cyclospora in captive chimpanzees and macaques by a quantitative PCR-based mutation scanning approach. Parasites and Vectors. (2015) 8:274. doi: 10.1186/s13071-015-0872-8
22. Zanzani, SA, Gazzonis, AL, Epis, S, and Manfredi, MT. Study of the gastrointestinal parasitic fauna of captive non-human primates (Macaca fascicularis). Parasitol Res. (2016) 115:307–12. doi: 10.1007/s00436-015-4748-9
23. Canelli, E, Luppi, A, Lavazza, A, Lelli, D, Sozzi, E, Moreno Martin, AM, et al. Encephalomyocarditis virus infection in an Italian zoo. Virol J. (2010) 7:1–7. doi: 10.1186/1743-422X-7-64
24. Poglayen, G, Varcasia, A, Bettini, G, Morandi, B, Galuppi, R, and Galliani, M. Echinococcus granulosus “sensu stricto” in a captive ring-tailed lemur (Lemur catta) in northern Italy. Pak Vet J. (2016) 36:121–3.
25. De Liberato, C, Berrilli, F, Meoli, R, Friedrich, KG, Di Cerbo, P, Cocumelli, C, et al. Fatal infection with Taenia martis metacestodes in a ring-tailed lemur (Lemur catta) living in an Italian zoological garden. Parasitol Int. (2014) 63:695–7. doi: 10.1016/j.parint.2014.05.008
26. Cavallero, S, De Liberato, C, Friedrich, KG, Di Cave, D, Masella, V, D’Amelio, S, et al. Genetic heterogeneity and phylogeny of Trichuris spp. from captive non-human primates based on ribosomal DNA sequence data. Infect Genet Evol. (2015) 34:450–6. doi: 10.1016/j.meegid.2015.06.009
27. Cavallero, S, Di Filippo, MM, Rondón, S, De Liberato, C, D’amelio, S, Friedrich, KG, et al. Nuclear and mitochondrial data on Trichuris from Macaca fuscata support evidence of host specificity. Life. (2021) 11:1–9. doi: 10.3390/life11010018
28. Montalbano Di Filippo, M, Meoli, R, Cavallero, S, Eleni, C, De Liberato, C, and Berrilli, F. Molecular identification of Mesocestoides sp. metacestodes in a captive gold-handed tamarin (Saguinus midas). Infect Genet Evol. (2018) 65:399–405. doi: 10.1016/j.meegid.2018.08.008
29. Botero, D, and Restrepo, M. Parasitosis humana. Quinta Medellín: Corporación para Investigaciones Biológicas (2012). 16 p.
30. Rondón, S, Cavallero, S, Link, A, González, C, and D’Amelio, S. Prevalence and molecular characterisation of Blastocystis sp. infecting free-ranging primates in Colombia. Pathogens. (2023) 12:1–8. doi: 10.3390/pathogens12040569
31. Rivero, J, Cutillas, C, and Callejón, R. Trichuris trichiura (Linnaeus, 1771) from human and non-human primates: morphology, biometry, host specificity, molecular characterization, and phylogeny. Front Vet Sci. (2021) 7:1–17. doi: 10.3389/fvets.2020.626120
32. Cavallero, S, Nejsum, P, Cutillas, C, Callejón, R, Dole, J, Modrý, D, et al. Insights into the molecular systematics of Trichuris infecting captive primates based on mitochondrial DNA analysis. Vet Parasitol. (2019) 272:23–30. doi: 10.1016/j.vetpar.2019.06.019
33. Kumar, S, Stecher, G, and Tamura, K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. (2016) 33:1870–4. doi: 10.1093/MOLBEV/MSW054
34. García-Sánchez, AM, Rivero, J, Callejón, R, Zurita, A, Reguera-Gomez, M, Valero, MA, et al. Differentiation of Trichuris species using a morphometric approach. Int J Parasitol Parasites Wildl. (2019) 9:218–23. doi: 10.1016/j.ijppaw.2019.05.012
35. Rivero, J, García-Sánchez, ÁM, Zurita, A, Cutillas, C, and Callejón, R. Trichuris trichiura isolated from Macaca sylvanus: morphological, biometrical, and molecular study. BMC Vet Res. (2020) 16:445–19. doi: 10.1186/s12917-020-02661-4
36. Callejón, R, Halajian, A, and Cutillas, C. Description of a new species, Trichuris ursinus n. sp. (Nematoda: Trichuridae) from Papio ursinus Keer, 1792 from South Africa. Infect Genet Evol. (2017) 51:182–93. doi: 10.1016/j.meegid.2017.04.002
37. Liu, D . Balantidium In: D Liu , editor. Molecular detection of human parasitic pathogens. Boca Raton: CRC Press (2013). 161–6.
38. Pomajbíková, K, Oborník, M, Horák, A, Petrželková, KJ, Grim, JN, Levecke, B, et al. Novel insights into the genetic diversity of Balantidium and Balantidium-like cyst-forming ciliates. PLoS Negl Trop Dis. (2013) 7:e2140. doi: 10.1371/journal.pntd.0002140
39. Secchioni, E, Sgorbini, M, and Perrucci, S. Gastrointestinal parasites, liver flukes and lungworms in domestic ruminants from Central Italy. Large Anim Rev. (2016) 22:195–201.
40. Giarratana, F, Nalbone, L, Napoli, E, Lanzo, V, and Panebianco, A. Prevalence of Balantidium coli (Malmsten, 1857) infection in swine reared in South Italy: a widespread neglected zoonosis. Vet World. (2021) 14:1044–9. doi: 10.14202/vetworld.2021.1044-1049
41. Stark, D, Barratt, J, Chan, D, and Ellis, JT. Dientamoeba fragilis, the neglected trichomonad of the human bowel. Clin Microbiol Rev. (2016) 29:553–80. doi: 10.1128/CMR.00076-15
42. Junaidi, CU, Purnawarman, T, Latif, H, Sudarnika, E, and Farida, M. The distribution of intestinal amoebae in wild long-tailed macaques (Macaca fascicularis) in Sabang city, Aceh Province, Indonesia. Trends Sci. (2022) 19:1–8. doi: 10.48048/tis.2022.1717
43. Ottino, L, Buonfrate, D, Paradies, P, Bisoffi, Z, Antonelli, A, Rossolini, GM, et al. Autochthonous human and canine Strongyloides stercoralis infection in Europe: report of a human case in an Italian teen and systematic review of the literature. Pathogens. (2020) 9:1–25. doi: 10.3390/pathogens9060439
44. Fuehrer, HP . An overview of the host spectrum and distribution of Calodium hepaticum (syn. Capillaria hepatica): part 2 - Mammalia (excluding Muroidea). Parasitol Res. (2014) 113:641–51. doi: 10.1007/s00436-013-3692-9
45. Foster, A, and Johnson, B. An explanation for the occurrence of Capillaria hepatica ova in human faeces suggested by the finding of three new hosts used as food. Trans R Soc Trop Med Hyg. (1939) 32:639–44. doi: 10.1016/S0035-9203(39)90027-1
46. Martin-Solano, S, Carrillo-Bilbao, GA, Ramirez, W, Celi-Erazo, M, Huynen, MC, Levecke, B, et al. Gastrointestinal parasites in captive and free-ranging Cebus albifrons in the Western Amazon, Ecuador. Int J Parasitol Parasites Wildl. (2017) 6:209–18. doi: 10.1016/j.ijppaw.2017.06.004
47. Hooshyar, H, Rostamkhani, P, Arbabi, M, and Delavari, M. Giardia lamblia infection: review of current diagnostic strategies. Gastroenterol Hepatol Bed Bench. (2019) 12:3–12.
48. Solórzano-García, B, and Pérez-Ponce de León, G. Parasites of neotropical primates: a review. Int J Primatol. (2018) 39:155–82. doi: 10.1007/s10764-018-0031-0
49. Albanese, V, Kuan, M, Accorsi, PA, Berardi, R, and Marliani, G. Evaluation of an enrichment programme for a colony of long-tailed macaques (Macaca fascicularis) in a rescue Centre. Primates. (2021) 62:585–93. doi: 10.1007/s10329-021-00908-8
Keywords: captive primates, intestinal parasites, Italy, molecular characterization, zoonosis
Citation: Rondón S, Cavallero S, Montalbano Di Filippo M, De Liberato C, Berrilli F, Capitani N and D’Amelio S (2024) Intestinal parasites infecting captive non-human primates in Italy. Front. Vet. Sci. 10:1270202. doi: 10.3389/fvets.2023.1270202
Edited by:
Vito Colella, The University of Melbourne, AustraliaReviewed by:
Andrew Thompson, Murdoch University, AustraliaStefania Perrucci, University of Pisa, Italy
Copyright © 2024 Rondón, Cavallero, Montalbano Di Filippo, De Liberato, Berrilli, Capitani and D’Amelio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvia Rondón, silvia.rondon@uniroma1.it
 Silvia Rondón
Silvia Rondón Serena Cavallero
Serena Cavallero Margherita Montalbano Di Filippo
Margherita Montalbano Di Filippo Claudio De Liberato
Claudio De Liberato Federica Berrilli
Federica Berrilli Nazareno Capitani5
Nazareno Capitani5  Stefano D’Amelio
Stefano D’Amelio