Abstract
Out-of-office blood pressure (BP) measurement is considered an integral component of the diagnostic algorithm and management of hypertension. In the era of digitalization, a great deal of wearable BP measuring devices has been developed. These digital blood pressure monitors allow frequent BP measurements with minimal annoyance to the patient while they do promise radical changes regarding the diagnostic accuracy, as the importance of making an accurate diagnosis of hypertension has become evident. By increasing the number of BP measurements in different conditions, these monitors allow accurate identification of different clinical phenotypes, such as masked hypertension and pathological BP variability, that seem to have a negative impact on cardiovascular prognosis. Frequent measurements of BP and the incorporation of new features in BP variability, both enable well-rounded interpretation of BP data in the context of real-life settings. This article is a review of all different technologies and wearable BP monitoring devices.
Similar content being viewed by others
Hypertension (HTN) is the most common risk factor for cardiovascular disease as it affects about one billion people worldwide and its prevalence is estimated to reach 1.5 billion by 2025 [1]. Notably, recent data from Italy and China show that cardiovascular mortality, was the leading cause of death even during the coronavirus disease (COVID-19) pandemic [2, 3].
Adequate blood pressure (BP) control, lower than new target-levels, is crucial towards improving cardiovascular prognosis [4]. Successful treatment is now based on protocol driven management and requires two basic components: first of all, early diagnosis and administration of appropriate treatment and lifestyle recommendations by physicians, and secondly, patients’ adherence to the treatment regimen and recommendations. However, despite the fact that many safe and effective drugs have been developed in the recent decades, HTN, as well as smoking, continues to be the main cause of cardiovascular morbidity and mortality worldwide, in both developed and developing countries. This is partly explained by frequently delayed diagnosis, but it can be also attributed to known limitations of diagnostic and therapeutic approaches applied to date, underscoring the necessity of new strategies to optimize the management of HTN.
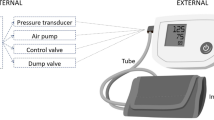
In latest years, out-of-office BP measurements have become essential component of BP control and this is clearly demonstrated in the recent guidelines that recommend the use of out-of-office measurements in order to optimize the management of hypertensive individuals [4, 5]. 24 h ambulatory BP monitoring (ABPM) and home BP monitoring (HBPM) are both acceptable methods of evaluating the hemodynamic burden in hypertensive patients in out-of-office setting. However, the number of measurements that can be recorded with these two methods is limited. In addition, ABPM has some limitations such as restricted availability and discomfort, particularly at night, while HBPM carries the risk of limited reliability and reproducibility. Therefore, wearable BP measuring device that provide office and out-of-office measurements (Fig. 1), could overcome all above limitations and could be really valuable in HTN management indeed, especially in the era of digitalization.
Methods of measuring BP
In 1998, Norman Kaplan wrote in an editorial of the American Journal of Hypertension: “The measurement of blood pressure is likely the clinical procedure of greatest importance that is performed in the sloppiest manner” – a statement which still is highly relevant [6]. For the time being, non-invasive BP monitors use the auscultatory and the oscillometric technique based on the use of cuff. The cuff is placed on the patient’s arm, and the cuff bladder is inflated with air until the external pressure exceeds the intra-arterial systolic pressure and arterial flow underneath the cuff ceases. The cuff bladder pressure is slowly released. The auscultatory method (also known as the Riva Rocci - Korotkoff or manual method for BP measurement) is the listening for Korotkoff sounds in the brachial artery. The oscillometric method is based on the principle that the point of maximal oscillations corresponds closely to mean arterial pressure and with an appropriate algorithm systolic and diastolic values are calculated. However, there are significant drawbacks in these methods. More specifically, cuff bladder monitoring devices cannot provide continuous BP measurements, as 1–2 min pause is required for both hemodynamic recovery and minimization of measurement errors [7]. Another limitation is that the measurement of BP depends on the adequate inflation of the cuff and the compression of the arm, which is energy-consuming and not easily tolerated by the patient. Ideally, the continuous monitoring of BP is a crucial factor concerning the diagnosis, management, and treatment of hemodynamic diseases such as HTN [8], but the capability of measuring BP, in this setting, is rather currently limited. Arterial catheterization is a common method of continuous BP monitoring, but this approach is invasive, costly, and used almost exclusively in an intensive care unit setting. Thus, a new strategy is required in order to measure BP, both continuously and non-invasively.
In the advanced digital healthcare information age, the approach to achieve this goal is shifting from the traditional methods (HBPM and ABPM) to wearable BP measuring devices. Wearable BP monitoring devices allow frequent BP measurements (ideally constant BP monitoring), with minimal discomfort. This novel technology is expected to drastically alter the way and the quality of detection and management of HTN, by providing a vast amount of BP data in different conditions, allowing more accurate diagnosis of phenotypes that have a negative impact on cardiovascular prognosis, such as masked HTN and pathological BP variability. Frequent BP measurements and the incorporation of new characteristics regarding BP variability, such as the simultaneous monitoring of environmental conditions, allows the interpretation and evaluation of BP data in relation to daytime activity, sleep periods and stress, in a real-life-time setting. This new digital approach in HTN is expected to significantly contribute in the field of preventive medicine, which refers to strategies designed to predict the occurrence of cardiovascular events and identify patients at increased cardiovascular risk, based on data collected over time, allowing interventions towards reducing this risk [9].
BP variability
The theory of synergistic resonance suggests that all forms of BP variability (beat-to-beat, 24 h, day-to-day, seasonal, annual) are able to generate dynamic increases in BP, which could coincide with BP peaks as a response to various external triggers (e.g., temperature, stress, sleep apnea, exercise) leading to increased occurrence of cardiovascular events, especially in patients with increased arterial stiffness and decreased arterial absorbance of hemodynamic fluctuations [10]. Therefore, there is a number of factors contributing towards a unique, tailored to each person, 24 h BP variability during daytime [11]. In addition, BP variability in hypertensive patients seems to vary, depending on the season, with higher peaks recorded during exercise in winter, probably due to lower temperature in the winter as compared to summer [12].
Many studies have demonstrated the strong association of BP variability with cardiovascular events. Excessive morning surge of BP has been correlated to increased risk of stroke, cerebral hemorrhage, and different hypertension-mediated organ damage (HMOD) [13, 14]. Moreover, both nocturnal HTN and non-dipping phenomenon of BP during nighttime in ABPM, are associated with higher risk of HMOD and cardiovascular events, in hypertensive and normotensive individuals [15]. This demonstrates the need for accurate detection of BP fluctuations in response to any external factor, as it is crucial to reducing cardiovascular risk. Wearable devices that can constantly record BP and evaluate all environmental factors (temperature, humidity, altitude, etc.) that have an impact on BP variability, may be profoundly effective in individualized hypertension control worldwide.
Wearable BP measuring devices
Recently, both scientific community and industry are interested in designing small, wearable devices to measure biological parameters and there has been a great effort in the digital device development process. These devices function using a variety of different approaches and technologies to monitor BP, while some of them have already been certified.
Oscillometric measurement at the wrist level
The basic principle behind this method is that when the pressure oscillations in a cuff sphygmomanometer are recorded during gradual depressurization, the point of maximum oscillation corresponds to the average intra -arterial pressure [16]. The oscillations start at about the level of systolic pressure and continue below the level of diastolic pressure, therefore the values of systolic and diastolic pressure may be evaluated indirectly only according to an empirical algorithm. This method has great advantages, as there is no need to place a transducer above the brachial artery and it is less sensitive to external noise (but not to low-frequency mechanical vibrations). The main disadvantage is that such recording devices do not work reliably during physical activity, as there is usually significant movement noise. This technique is widely implemented in the existing validated BP measuring devices used for office, HBPM, and ABPM [17].
The new devices with wrist-cuff developed in the last years use the same technique, but it is expected to cause less compression of muscles and discomfort to the patient than the traditional arm-cuff. In a recent study [18], a clock-sized wrist BP measuring device was certified based on the protocol of the American National Standards Institute, Inc./Association for the Advancement of Medical Instrumentation/International Organization for Standardization (ANSI/AAMI/ISO) 81060-2:2013. The device used in the trial was the Omron HEM‐6410 T (HeartGuide – Omron Healthcare Co., Ltd) which has an extremely rigid inflatable belt to make an oscillometric BP measurement in the same way as a regular cuff sphygmomanometer and it is available in two sizes (ZM and ZL), depending on the size of the wrist. The small size of the device and the clock-like display, make it suitable for everyday use, providing multiple measurements in the daily work environment, in stressful conditions and even during sleeping periods. It takes on-demand measurements but can also be programmed for specific measurements even during sleep. Depending on the use, the battery lasts from 10 to 14 days on a single charge, which facilitates its use by elderly patients. There is certainly, the restriction that patients should place their wrist at the level of the heart for accurate measurement, while the device was certified only in sitting position and not in lying or standing position. A recent study has shown that BP deviates by 7 mmHg if the difference between the height of the heart and the position of the cuff is 10 cm, due to hydrostatic pressure [19]. Therefore, it is anticipated that future large-scale trials will evaluate the accuracy of this watch-type device under real-life conditions, during daytime and night periods, and investigate the interpretation of the measurements.
In a recent head-to-head study, it was for the first time that the oscillometric BP measuring wrist device (HEM-6410T, HeartGuide – Omron Healthcare Co., Ltd.) was compared to a 24 h ambulatory BP measuring device [20]. After conducting continuous measurements both in office and out-of-office setting in 50 patients, there were comparable results between the two arms. The Heart Guide device showed only minor deviations in out-of-office measurements of 3.2 ± 17.0 mm Hg (p < 0.001) in systolic BP (SBP) and −3.2 ± 11.3 mm Hg in diastolic BP (DBP) (p < 0.001), attributed mainly to the lower position of the wrist. The percentage of differences that were within the ± 10 mm Hg range was 58.7% in the office and 47.2% in the out-of-office measurements.
BP measurements using oscillometric finger cuffs (Method of Penaz)
Arterial pulse in the finger is detected by a photoplethysmographic sensor, located underneath a finger cuff. The output of the plethysmograph is used to guide a pump, which quickly moderates the cuff pressure so that the artery is kept partially open. The pressure oscillations of the cuff are recorded and it is proven that they resemble the intra-arterial pressure wave fluctuations in most individuals. This method provides an accurate estimate of changes in systolic and diastolic pressure compared to brachial artery pressure [21]. The cuff can be kept inflated for up to 2 h. Commercially available devices Finometer and Portapres can record pressure oscillations and have been certified in several studies comparing finger and intra-arterial BP monitoring [22, 23]. Furthermore, the device Portapres allows 24 h ambulatory BP recording, though there are certain difficulties regarding the size and the complexity of the device [24].
Applanation Tonometry method
The constant and continuous BP monitoring with a device based on arterial tonometry was first implemented in 1963 [25]. Arterial tonometry is a method that allows noninvasively BP measurement within a superficial artery, located in close contact with a solid bone structure. The radial artery is the most commonly used, because of its large diameter and accessibility. The sensor is compressed on the artery through the pressure of a hemispherical air-chamber. The optimum pressure is automatically applied to flatten (but not occlude) a portion of the arterial wall and maximize the pulse pressure measured by tension transducers lying in contact with the artery. The endo-aortic pressure can be measured by the transducer, as the arterial wall stress is considered negligible since the artery is flattened. The oscillometric measurement of the systolic and diastolic BP values take place during continuous and steady decompression of the air chamber [26].
The accuracy of the method is quite questionable, as it largely depends on the location of the artery and the changes in the contact force required to keep the artery in a state of partial compression. The sensor should be well-positioned, in a fixed state above the artery during BP measurement. Devices using this technology are certified according to the ESH (European Society of Hypertension) protocol and AAMI (Association for the Advancement of Medical Instrumentation) standards. The Health STATS International (Singapore) device has shown satisfactory results, overestimating SBP by ≤1.3 mm Hg and underestimating DBP by ≤4.6 mm Hg. However, the measurements were more reliable in the sitting and lying position rather than the standing position [27]. This is also reflected in the reduced accuracy of ambulatory measurements [28], especially in patients with vessels with increased calcium load such as patients with chronic kidney disease [29]. Another limitation is that the position relationship between the heart and the measuring point varies, which introduces the effect of hydrostatic pressure changes.
Devices based on the applanation tonometry method have been used to monitor nocturnal BP and detect hypertensive peaks, especially in patients with sleep apnea syndrome [30], and an algorithm has been developed for the detection of pathological BP peaks (lasting seconds), based on beat-to-beat BP measurements during nighttime [31].
Photoplethysmography
Photoplethysmography (PPG) is a non-invasive technique that appeared in 1930s, through which changes in blood flow are detected in selected parts of the body during the cardiac cycle. Clinical applications of this technique are found in the assessment of BP, heart rate and blood oxygen saturation, but also in the detection of peripheral venous diseases.
Pulse transit time (PTT), indicating the time it takes for a pulse wave to travel along the length of the arterial tree, is an essential part of measuring BP with this method. The pulse pressure wave form, occurs when blood is ejected from the left ventricle and the impulse transmitted to the arterial wall moves at greater velocity than the blood itself. In addition to measuring BP, PTT is an index of arterial stiffness. The PTT may be derived from calculations on signals from the electrocardiogram (ECG) and PPG. It is based on strictly defined characteristics of the shape of the pulse pressure waveforms in blood vessels.
ECG data is used as a basis for calculating time, while PPG provides a visual evaluation of the volumetric changes of blood in the tissues during the cardiac cycle. Optical PPG and ECG sensors are already used in wearable devices for measuring heart rate. The PTT measurement involves calculating the time between the R wave on the ECG and a reference point in the pulse pressure wave measured using PPG, providing data on the levels of BP [32]. Nevertheless, PTT-based BP estimation may not be accurate enough as BP regulation is a multifactorial process. Studies have shown that measuring PTT alone is not enough in terms of measurement reliability. The integration of PPT, heart rate and one recent conventional BP measurement in the equation could increase the reliability of the BP evaluation [33].
Recently there were developed BP measurement devices using multiple PPG sensors without requiring incorporation of PTT. A wrist-size device (smartWatch, CareUp®) with two PPG sensors, has been proved to be quite accurate compared to conventional oscillometric devices, but without reaching AAMI certification prerequisites [34]. This method has two main limitations. Firstly, the stability of the PPG signal is affected by movements, narrowing its use to only non-ambulatory setting. A second limitation is the need for frequent calibration of the device. The device is primary calibrated by the doctor at the office; in this model it is considered that the heart rate does not change over time, taken into account a mean heart rate for the calibration of the device. However, hypertensive individuals seem to have high heart rate variability, therefore periodic calibration of the device is demanded.
Although many studies have investigated the use of multi- sensor devices based on PPG in BP measurement, a systematic review and meta-analysis demonstrated that there is still insufficient data to evaluate these devices regarding reliable non-invasive BP recording [35].
Integrating photoplethysmography in smartphone applications
Recently conducted studies investigated the efficacy of BP measurement using PPG signals via a smartphone camera or integrated into a portable detector connected to a smartphone. In a single study, 205 individuals were enrolled and data from PPG signals were collected, using the heart rate sensor of smartphone Samsung Galaxy S6 [36]. In addition, an algorithm was developed based on the demographic characteristics of the patients that improved the recording accuracy by 11.5% regarding SBP and 18% regarding DBP. Based on PPG signals and this integrated algorithm, there were measurements achieved with accuracies of 7 mmHg mean absolute error for SBP, and 5 mmHg for DBP.
In another trial, a pressure sensor was incorporated in the PPG sensor of a smartphone [37]. In 2 of the 30 subjects of the study no BP measurement was achieved, while 60% of the measurements were not successful. In the analysis of the accuracy of the measurements compared to the conventional oscillometric BP measurement method, an average accuracy variation of 8.8 mmHg for SBP and 7.7 mmHg for DBP was achieved.
An innovative, proof-of-concept study utilized PPG for measuring BP without physical contact, by depicting blood flow from facial videos [38]. The researchers used transdermal optical imaging technology from a mobile phone camera to reveal blood flow profile and calculate the levels of BP in normotensive individuals by using a developed algorithm. BP values were estimated with the assistance of this algorithm, with an accuracy of 94.8% regarding SBP and 95.7% regarding DBP. There are serious limitations on this study, since enrolled only Asian normotensive individuals, and the trial took place in laboratory conditions under special lighting setting. The accuracy of measurements in clinical conditions may differ because of the changes in environmental conditions, camera angle and distance, skin color, and facial features.
Directions for the future
The Institute of Electrical and Electronics Engineers (IEEE) has proposed a certification protocol for new generation measuring BP devices, in order to overcome issues associated with the use of certification protocols developed for cuff-based devices [39]. This protocol requires the same reliability and accuracy with AAMI standardization for the conventional devices. The IIEE protocol differs from the AAMI/ESH protocols in the inclusion of measurements even after evoked changes of BP in the studied subjects, after initial calibration, to ensure the accuracy of measurements in a broad range of BP values. In addition, the IEEE protocol includes measurements taken over an extended period (weeks to months) after the initial calibration to investigate the stability of long-term calibration.
At the same time, the development of new technologies and systems of telecommunication [40] provides doctors with access to out-of-office BP measurements through remote BP monitoring programs, thereby promoting a more effective patient-physician relationship. Remote BP monitoring strategy has been shown to be effective in reducing BP and encountering both physicians’ inertia and poor patients’ compliance; [41,42,43,44] when combined with novel BP monitoring techniques, may radically improve personalized management of hypertensive patients.
The development of new BP recording devices has been a highly active field of research in improving measurement accuracy, using a variety of technologies (Table 1). However, even if accurate and user-friendly smartphone applications and wearable cuffless devices will be developed in the near future, there are several steps that should be considered prior to implementation in clinical practice. First of all, scientific societies should introduce protocols to validate the cuffless devices. Currently, there is no protocol for the validation of these devices, while some of them have used the protocol of traditional cuff bladder monitoring devices, which is probable not appropriate for the validation of cuffless devices. The cuffless devices needs calibration not only at initial level but in many time frames, at several BP levels and at both static and dynamic states. Specific validation protocols should be designed at three levels: (a) static test, (b) test with BP change from the calibration point, and (c) test after a certain period of time from calibration. Moreover, if used for remote BP monitoring, there should be a safe and efficient storage of data that respects the personal data protection law and is accessible for health providers. Then, large-scale prospective clinical studies should be carried out in order to evaluate what BP values recorded by these devices should be considered as “normal” in daily life, and what BP values – or range of values – are associated with adverse events. Finally, the implementation of remote BP monitoring with cuffless devices in daily clinical HTN management, requires the appropriate financial compensation for the involved health providers.
Conclusion
All available evidence suggests that the digital management of HTN and the wearable BP monitoring devices are considered the technology of the future. These approaches provide a realistic prospect concerning the goal of reducing or even eliminating cardiovascular events in hypertensive patients. The availability of simple and affordable devices for reliable cuffless BP measurement allows the diagnosis of HTN at an early stage, improves BP control, but also significantly increases the use of remote monitoring in the management of HTN. Despite the promising development and preliminary encouraging results, most of the current wearable BP measuring devices have not even been certified and have limited use in clinical practice due to problems concerning the essential frequent calibration and the dependence of reliable measurements only in specific body positions. As the field of biotechnology continues to expand, further research is needed to improve current BP measurement technologies utilized cuffless devices. The next important question is how we will implement these new technologies into clinical practice in the best way for both physicians and patients.
Data availability
The data generated or analysed during this study can be found within the published article and its supplementary.
References
Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–50.
COVID-19 Surveillance Group Characteristics of COVID-19 Patients dying in Italy Report Based on Available Data on 24 March 2020; The Italian National Health Service: Rome, Italy, 27 March 2020.
World Health Organization (WHO) -China Joint Mission. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19); World Health Organization (WHO): Geneva, Switzerland, 28 February 2020.
Williams B, Mancia G, Spiering W, Rosei EA, Azizi M, Burnier M, et al. ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;2018:3021–104.
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Himmelfarb CD, et al. ACC / AHA / AAPA / ABC / ACPM / AGS /APhA/ ASH / ASPC / NMA / PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology / American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;00:e000–e000.
Kaplan NM. Commentary on the sixth report of the Joint National Committee (JNC-6). Am J Hypertens. 1998;11:134–6.
Choi YM, Leopold D, Campbell K, Mulligan J, Grudic GZ, Moulton SL. Noninvasive monitoring of physiologic compromise in acute appendicitis: New insight into an old disease. J Pediatr Surg. 2018;53:241–6.
Ding XR, Zhao N, Yang GZ, Pettigrew RI, Lo B, Miao F, et al. Continuous blood pressure measurement from invasive to unobtrusive: celebration of 200th birth anniversary of carl ludwig. IEEE J Biomed Health Inf. 2016;20:1455–65.
Kario K. Management of hypertension in the digital era: small wearable monitoring devices for remote blood pressure monitoring. Hypertension 2020;76:640–50.
Kario K. New insight of morning blood pressure surge into the triggers of cardiovascular disease-synergistic resonance of blood pressure variability. Am J Hypertens. 2016;29:14–16.
Kario K, Chirinos JA, Townsend RR, Weber MA, Scuteri A, Avolio A, et al. Systemic hemodynamic atherothrombotic syndrome (SHATS) - coupling vascular disease and blood pressure variability: proposed concept from pulse of Asia. Prog Cardiovasc Dis. 2020;63:22–32.
Kario K, Tomitani N, Kanegae H, Yasui N, Nishizawa M, Fujiwara T, et al. Development of a new ICT-based multisensory blood pressure monitoring system for use in hemodynamic biomarker-initiated anticipation medicine for cardiovascular disease: the National IMPACT Program Project. ProgCardiovascDis. 2017;60:435–49.
Kuwajima I, Mitani K, Miyao M, Suzuki Y, Kuramoto K, Ozawa T. Cardiac implications of the morning surge in blood pressure in elderly hypertensive patients: relation to arising time. Am J Hypertens. 1995;8:29–33.
Yano Y, Hoshide S, Inokuchi T, Kanemaru Y, Shimada K, Kario K. Association between morning blood pressure surge and cardiovascular remodeling in treated elderly hypertensive subjects. Am J Hypertens. 2009;22:1177–82.
Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183–9.
Mauck GW, Smith CR, Geddes LA, Bourland JD. The meaning of the point of maximum oscillations in cuff pressure in the indirect measurement of blood pressure - part ii. J Biomech Eng. 1980;102:28–33.
Arakawa T. Recent research and developing trends of wearable sensors for detecting blood pressure. Sensors. 2018;18:2772. 23
Kuwabara M, Harada K, Hishiki Y, Kario K. Validation of two watch-type wearable blood pressure monitors according to the ANSI / AAMI / ISO81060-2: 2013 guidelines: Omron HEM-6410T-ZM and HEM-6410T-ZL. J Clin Hypertens. 2019;21:853–8.
Kikuya M, Chonan K, Imai Y, Goto E, Ishii M. Research group to assess the validity of automated blood pressure measurement devices in Japan. Accuracy and reliability of wrist ‐ cuff devices for self ‐ measurement of blood pressure. J Hypertens. 2002;20:629–38.
Kario K, Shimbo D, Tomitani N, Kanegae H, Schwartz JE, Williams B. The first study comparing a wearable watch-type blood pressure monitor with a conventional ambulatory blood pressure monitor on in-office and out-of-office settings. J Clin Hypertens. 2020;22:135–41.
Penaz J Photo-electric measurement of blood pressure, volume and flow in the finger. In Proceedings of the Digest of the Tenth International Conference on Medical Biological Engineering, Dresden, Germany, 13–17 August 1973.
Parati G, Casadei R, Groppelli A, Di Rienzo M, Mancia G. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension. 1989;13:647–55. 6 Pt 1.
Van Egmond J, Hasenbos M, Crul JF. Invasive v. non-invasive measurement of blood pressure. Comparison of two automatic methods and simultaneously measured direct intra-arterial pressure. Br J Anaesth. 1985;57:434–44.
Imholz BP, Langewouters GJ, van Montfrans GA, Parati G, van Goudoever J, Wesseling KH, et al. Feasibility of ambulatory, continuous 24-hour finger arterial pressure recording. Hypertension. 1993;21:65–73.
Pressman GL, Newgard PM. A transducer for the external measurement of arterial blood pressure. IEEE Trans Biomed Eng. 1963;10:73–81.
Sato T, Nishinaga M, Kawamoto A, Ozawa T, Takatsuji H. Accuracy of a continuous blood pressure monitor based on arterial tonometry. Hypertension. 1993;21:866–74.
Nair D, Tan SY, Gan HW, Lim SF, Tan J, Zhu M, et al. The use of ambulatory tonometric radial arterial wave capture to measure ambulatory blood pressure: the validation of a novel wrist-bound device in adults. J Hum Hypertens. 2008;22:220–2.
Komori T, Eguchi K, Hoshide S, Williams B, Kario K. Comparison of wrist-type and arm-type 24-h blood pressure monitoring devices for ambulatory use. Blood Press Monit. 2013;18:57–62.
Hornstrup BG, Rosenbæk JB, Bech JN. Comparison of ambulatory tonometric and oscillometric blood pressure monitoring in hypertensive patients. Integr Blood Press Control. 2020;13:41–47.
Kario K. Evidence and perspectives on the 24-hour management of hypertension: hemodynamic biomarker-initiated ‘anticipation medicine’ for zero cardiovascular event. Prog Cardiovasc Dis. 2016;59:262–81.
Kokubo A, Kuwabara M, Nakajima H, Tomitani N, Yamashita S, Shiga T, et al. Automatic detection algorithm for establishing standard to identify “surge blood pressure”. Med Biol Eng Comput. 2020;58:1393–404.
Van Velzen MHN, Loeve AJ, Niehof SP, Mik EG. Increasing accuracy of pulse transit time measurements by automated elimination of distorted photoplethysmography waves. Med Biol Eng Comput. 2017;55:1989–2000.
Wang R, Jia W, Mao ZH, Sclabassi RJ, Sun M. Cuff-free blood pressure estimation using pulse transit time and heart rate. Int Conf Signal Process Proc. 2014;2014:115–8.
Lazazzera R, Belhaj Y, Carrault G. A new wearable device for blood pressure estimation using photoplethysmogram. Sensors. 2019;19:2557.
Chan G, Cooper R, Hosanee M, Welykholowa K, Kyriacou PA, Zheng D, et al. Multi-site photoplethysmography technology for blood pressure assessment: challenges and recommendations. J Clin Med. 2019;8:1827.
Dey J, Gaurav A, Tiwari VN. InstaBP: cuff-less blood pressure monitoring on smartphone using single PPG sensor. Annu Int Conf IEEE Eng Med Biol Soc 2018;2018:5002–5.
Chandrasekhar A, Kim CS, Naji M, Natarajan K, Hahn JO, Mukkamala R. Smartphone-based blood pressure monitoring via the oscillometric finger-pressing method. Sci Transl Med. 2018;10:eaap8674.
Luo H, Yang D, Barszczyk A, Vempala N, Wei J, Wu SJ, et al. Smartphone-based blood pressure measurement using transdermal optical imaging technology. CircCardiovasc Imaging. 2019;12:e008857.
IEEE 1708-2014 - IEEE Standard for Wearable Cuffless Blood Pressure Measuring Devices. Available online at: https://standards.ieee.org/findstds/standard/1708-2014.html.
Michalakeas C, Katsi V, Soulaidopoulos S, Dilaveris P, Vrachatis D, Lekakis I, et al. Mobile phones and applications in the management of patients with arterial hypertension. Am J CardiovascDis. 2020;10:419–31.
Omboni S, Gazzola T, Carabelli G, Parati G. Clinical usefulness and cost effectiveness of home blood pressure telemonitoring: meta-analysis of randomized controlled studies. J Hypertens. 2013;31:455–67.
Pellaton C, Vybornova A, Fallet S, Marques L, Grossenbacher O, De Marco B, et al. Accuracy testing of a new optical device for noninvasive estimation of systolic and diastolic blood pressure compared to intra-arterial measurements. Blood Press Monit. 2020;25:105–9. https://doi.org/10.1097/MBP.0000000000000421.
Vybornova A, Polychronopoulou E, Wurzner-Ghajarzadeh A, Fallet S, Sola J, Wuerzner G. Blood pressure from the optical Aktiia Bracelet: a 1-month validation study using an extended ISO81060-2 protocol adapted for a cuffless wrist device. Blood Press Monit. 2021;26:305–11.
Dörr M, Weber S, Birkemeyer R, Leonardi L, Winterhalder C, Raichle CJ, et al. iPhone App compared with standard blood pressure measurement -The iPARR trial. Am Heart J. 2021;233:102–8.
Author information
Authors and Affiliations
Contributions
KT (K Tsioufis) was responsible for designing the rationale of the article. FT, KT (K Thomopoulos), KD, DT were responsible for conducting the search, screening potentially eligible studies, extracting and analysing data, interpreting results, and updating reference lists. DK and PI were responsible for collecting all above data according to the article rationale and write the main article as well as designing the table and figure of the article
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Konstantinidis, D., Iliakis, P., Tatakis, F. et al. Wearable blood pressure measurement devices and new approaches in hypertension management: the digital era. J Hum Hypertens 36, 945–951 (2022). https://doi.org/10.1038/s41371-022-00675-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-022-00675-z
This article is cited by
-
7-day Aktiia bracelet vs 24 h ABPM study: more questions than answers?
Hypertension Research (2023)